Application of mycoplasma bovis MbovP730 protein in natural infection and vaccine immunity identification
A technology of Mycoplasma bovis, antigenic protein, applied in chemical instruments and methods, microorganism-based methods, biochemical equipment and methods, etc., can solve the problems of cattle industry losses, poor effects, long clinical treatment cycles, etc. original effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
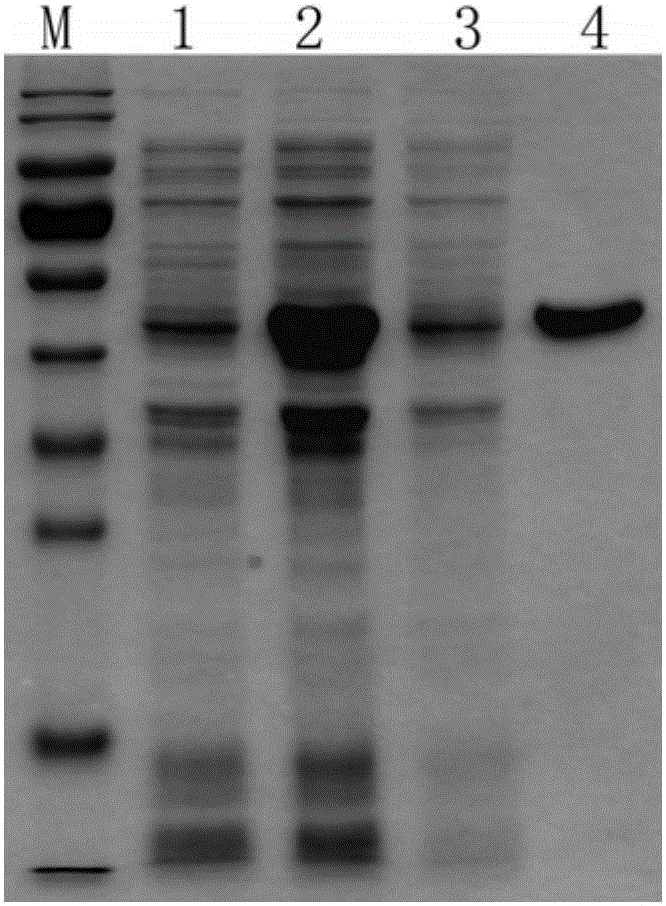
[0037] Embodiment 1: Cloning expression of Mycoplasma bovis Mbov_730 protein
[0038] 1.1 Cloning and expression of Mycoplasma bovis Mbov_730 gene
[0039] Due to the codon preference of Escherichia coli, the codon UGA encoding tryptophan in Mycoplasma bovis is used as a terminator in Escherichia coli, therefore, when expressing the Mbov_730 gene of Mycoplasma bovis with Escherichia coli, the Mycoplasma Mbov_730 gene needs to be mutated, That is, the codon UGA of Mycoplasma bovis was mutated to the codon UGG of tryptophan which can be expressed in Escherichia coli.
[0040] The applicant named the Escherichia coli obtained after mutation as Escherichia coli pET-30-Mbov-730, Escherichia coli, pET-30-Mbov-730, and sent it to China. Wuhan. Wuhan University, China for the preservation of typical cultures on December 18, 2015 Central deposit, deposit number is CCTCC NO: M2015762.
[0041] In this example, PCR primers were used to mutate the corresponding genes. The initial strai...
Embodiment 2
[0078] Embodiment 2: Mycoplasma bovis rMbovP730 protein specific detection
[0079] 2.1 Preparation of polyclonal antibody
[0080] Using the rMbovP730 protein prepared above to immunize BALB / C mice, the immunization procedure is as follows:
[0081] (1) For the first immunization, Mycoplasma bovis rNOX antigen 100 μg / mouse, plus Freund's complete adjuvant, subcutaneously injected multiple points on the back of the neck, 0.2mL / mouse.
[0082] (2) Two weeks later, for the second immunization, the dose of Mycoplasma bovis rMbovP730 antigen was 100 μg / mouse, and Freund's incomplete adjuvant was added to subcutaneously inject multiple points on the back of the neck.
[0083] (3) Four weeks later, for the third immunization, the dose of Mycoplasma bovis rMbovP730 antigen was 100 μg / rat, and Freund's incomplete adjuvant was added to subcutaneously inject multiple points on the back of the neck.
[0084] (4) After 7 days, cut the tip of the tail to collect blood, separate the serum...
Embodiment 3
[0087] Embodiment 3: the application of Mycoplasma bovis MbovP730 protein in distinguishing natural infection of Mycoplasma bovis field strain and immunity of Mycoplasma bovis vaccine strain
[0088] 3.1 Using M.bovis HB0801 whole bacterial protein as the coating antigen to detect serum by indirect ELISA method
[0089] (1) Animal serum sample collection: Mycoplasma bovis wild strain natural infection serum comes from clinical trials of Mycoplasma bovis suspected infected cattle, Mycoplasma bovis vaccine strain Mbov HB0801-150.2 (namely Mycoplasma bovis Mbov HB0801-150.2) (in vitro by M.bovisHB0801 Inoculation of PPLO liquid medium, 3 days as one generation, continuous passage in vitro for 150 generations) The immune serum comes from clinical trials, and the negative serum comes from the blank group of clinical immune experiments.
[0090] (2) Preparation of whole bacterial protein: M.bovis HB0801 on PPLO medium (preparation method: take 10.5g of PPLO powder, 2.5g of yeast pow...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com