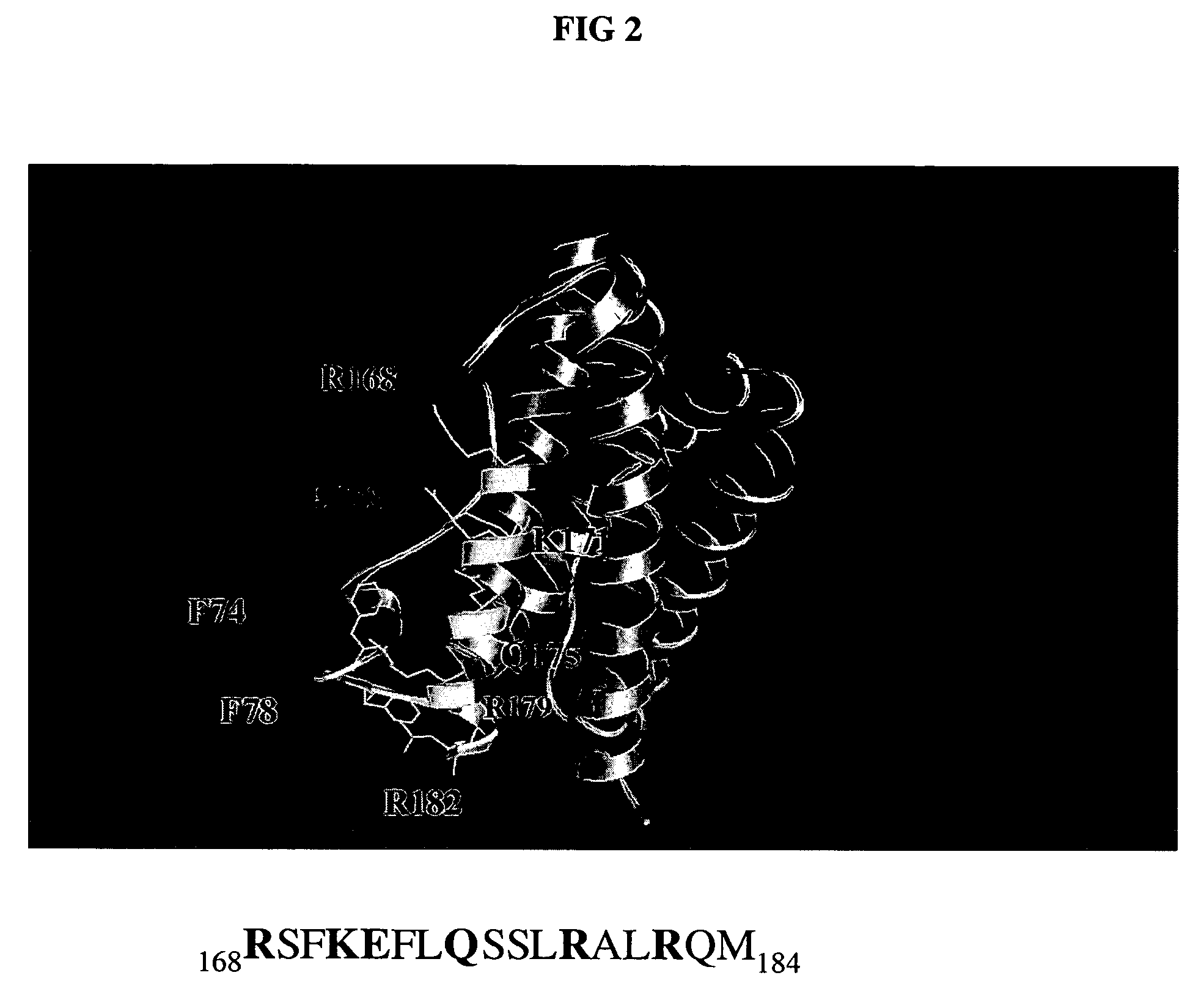
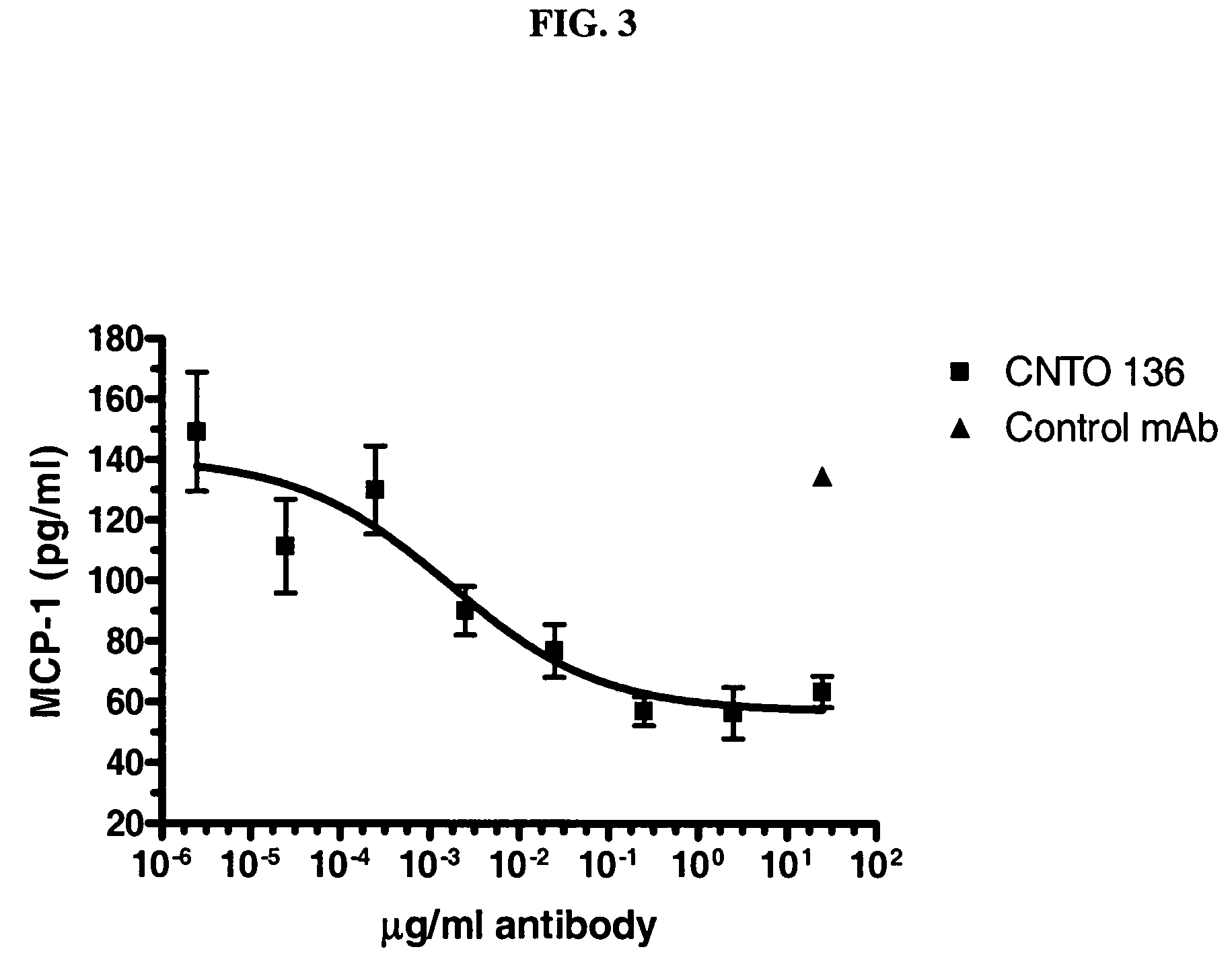
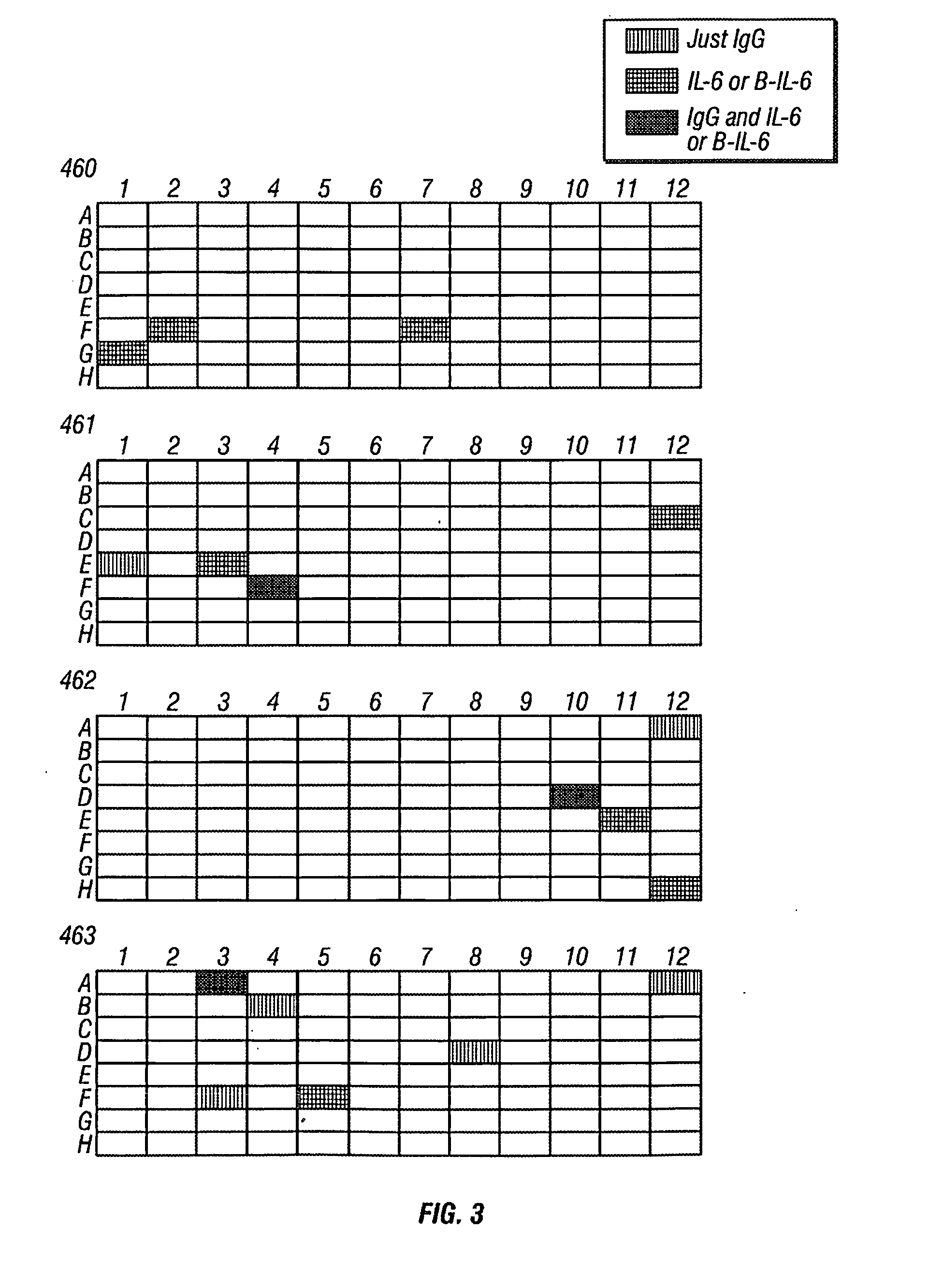
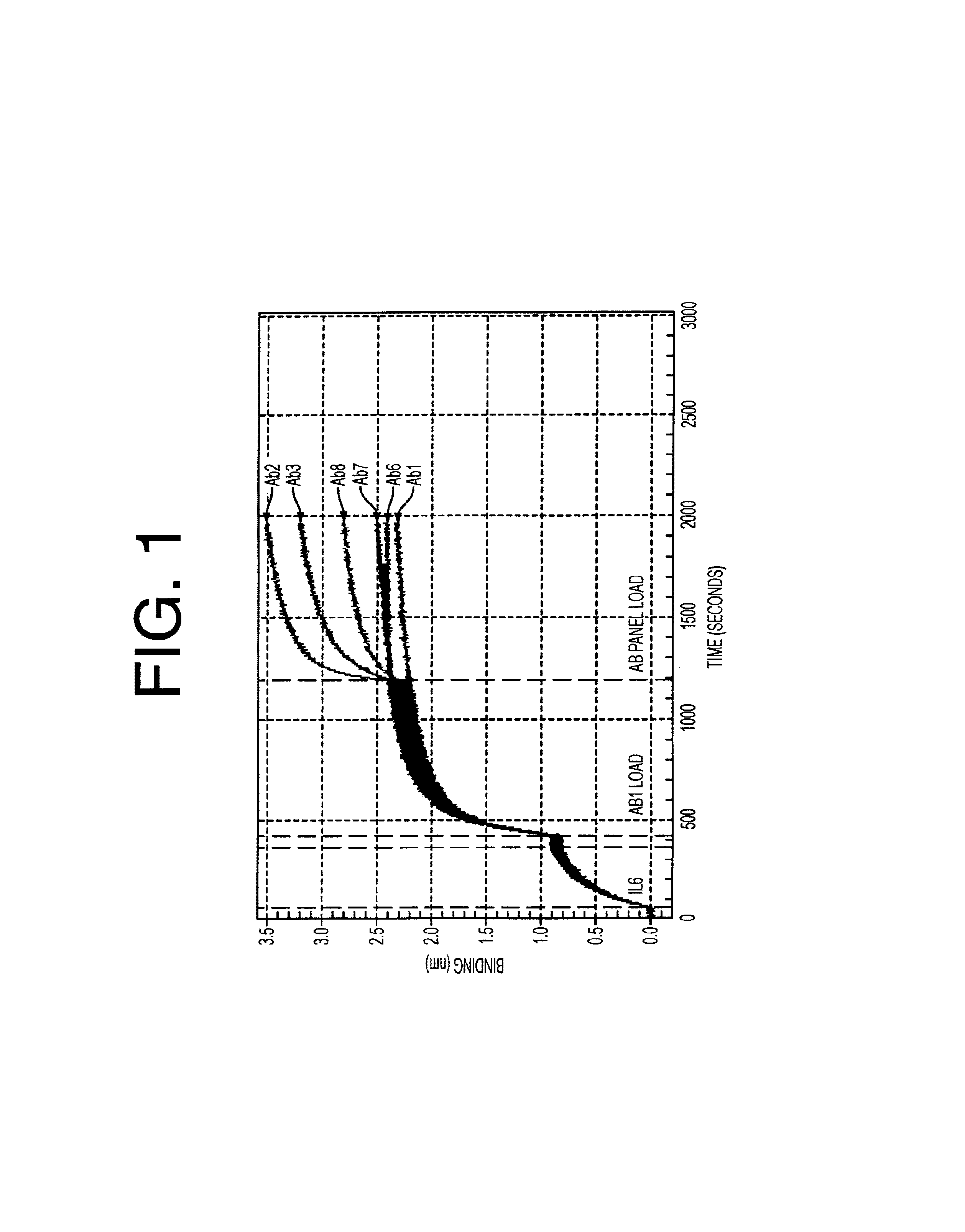
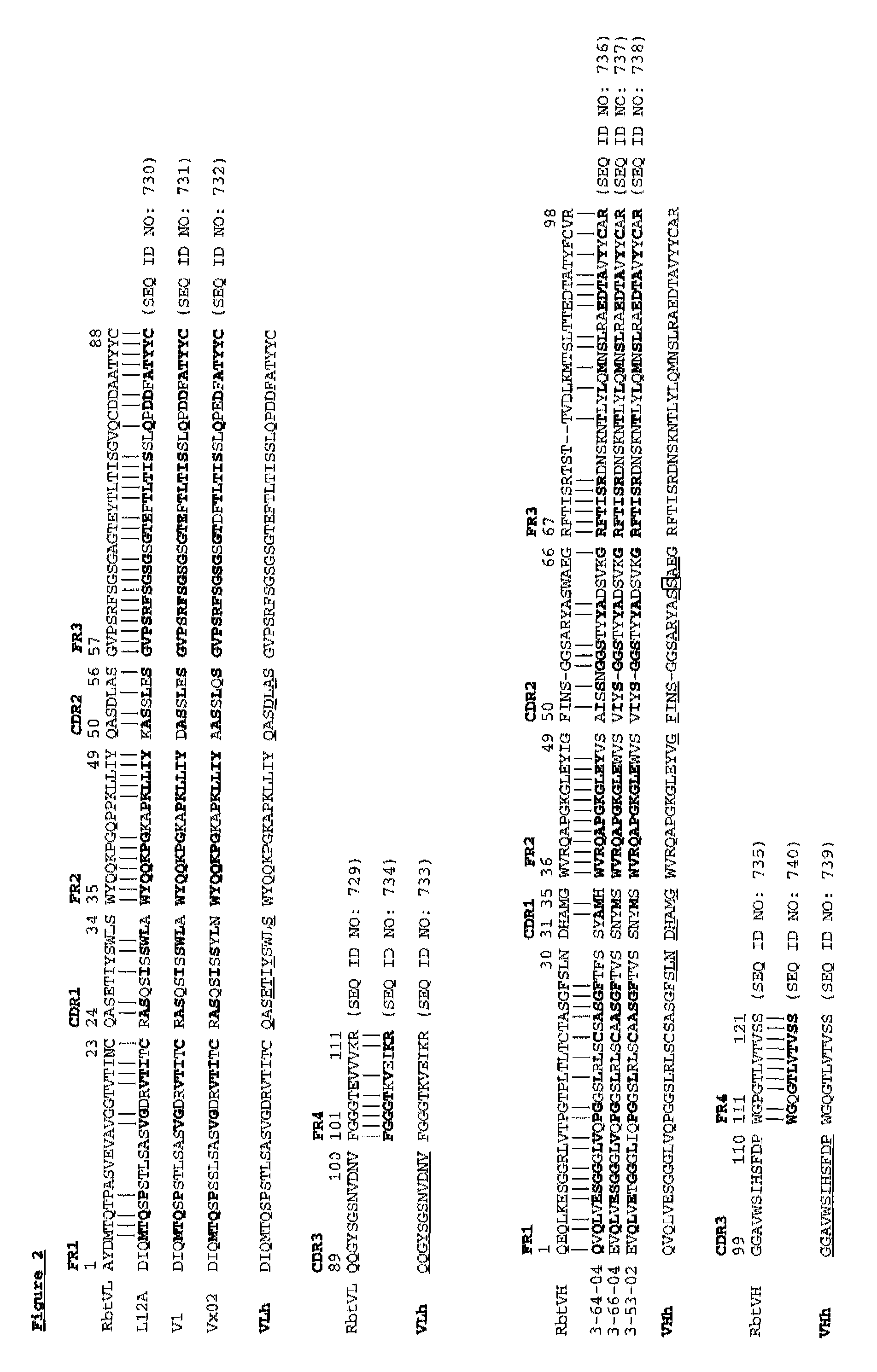
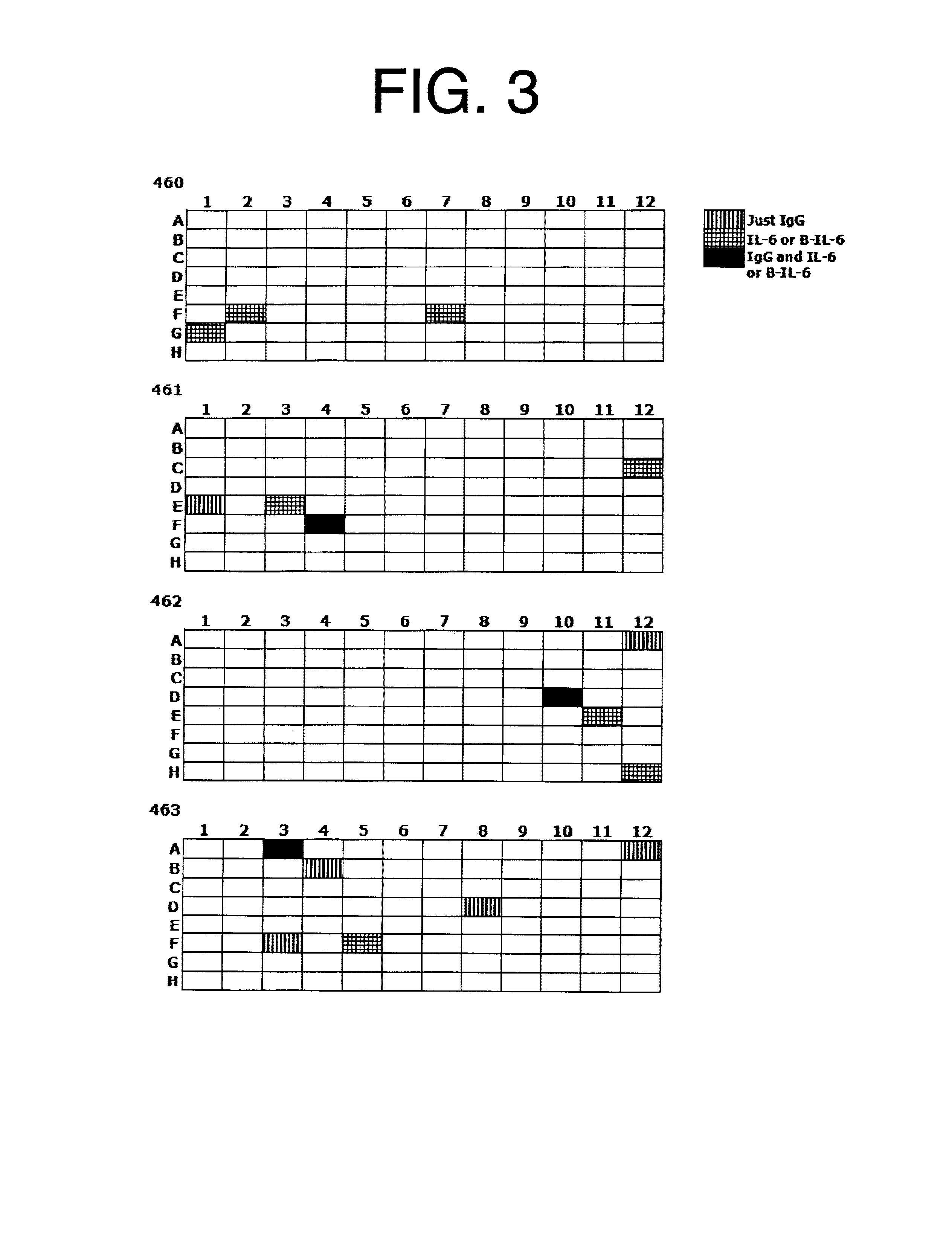
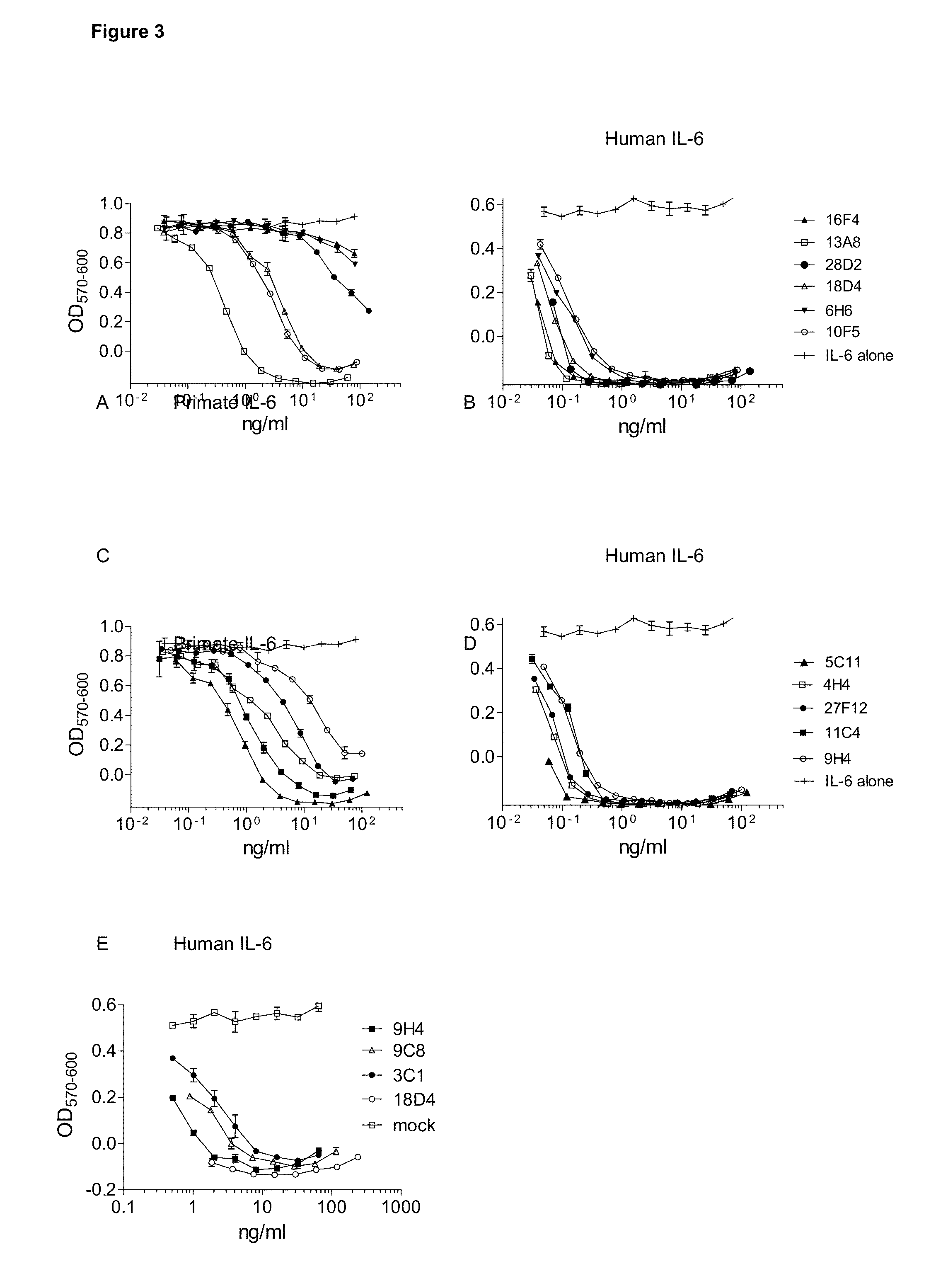
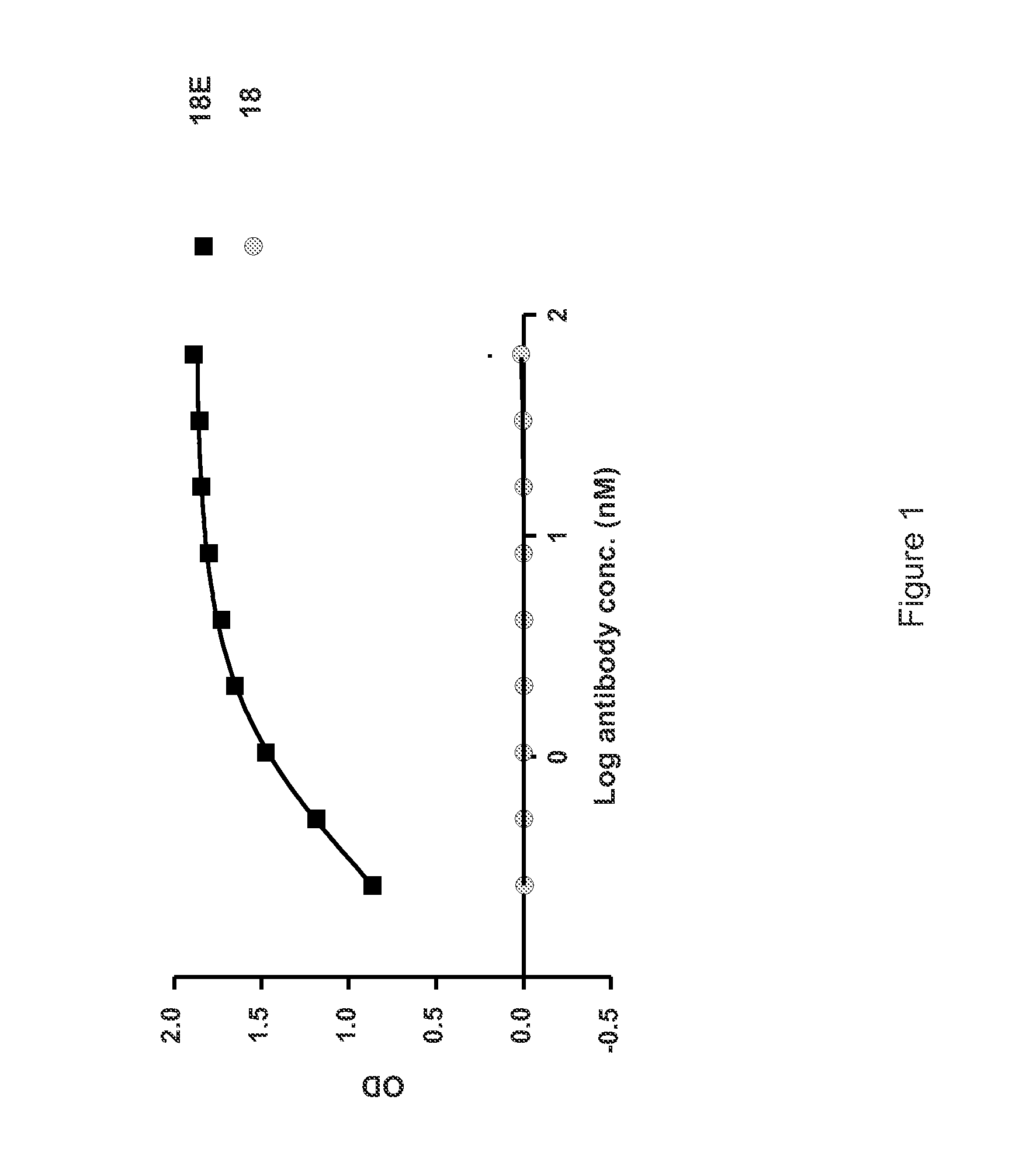
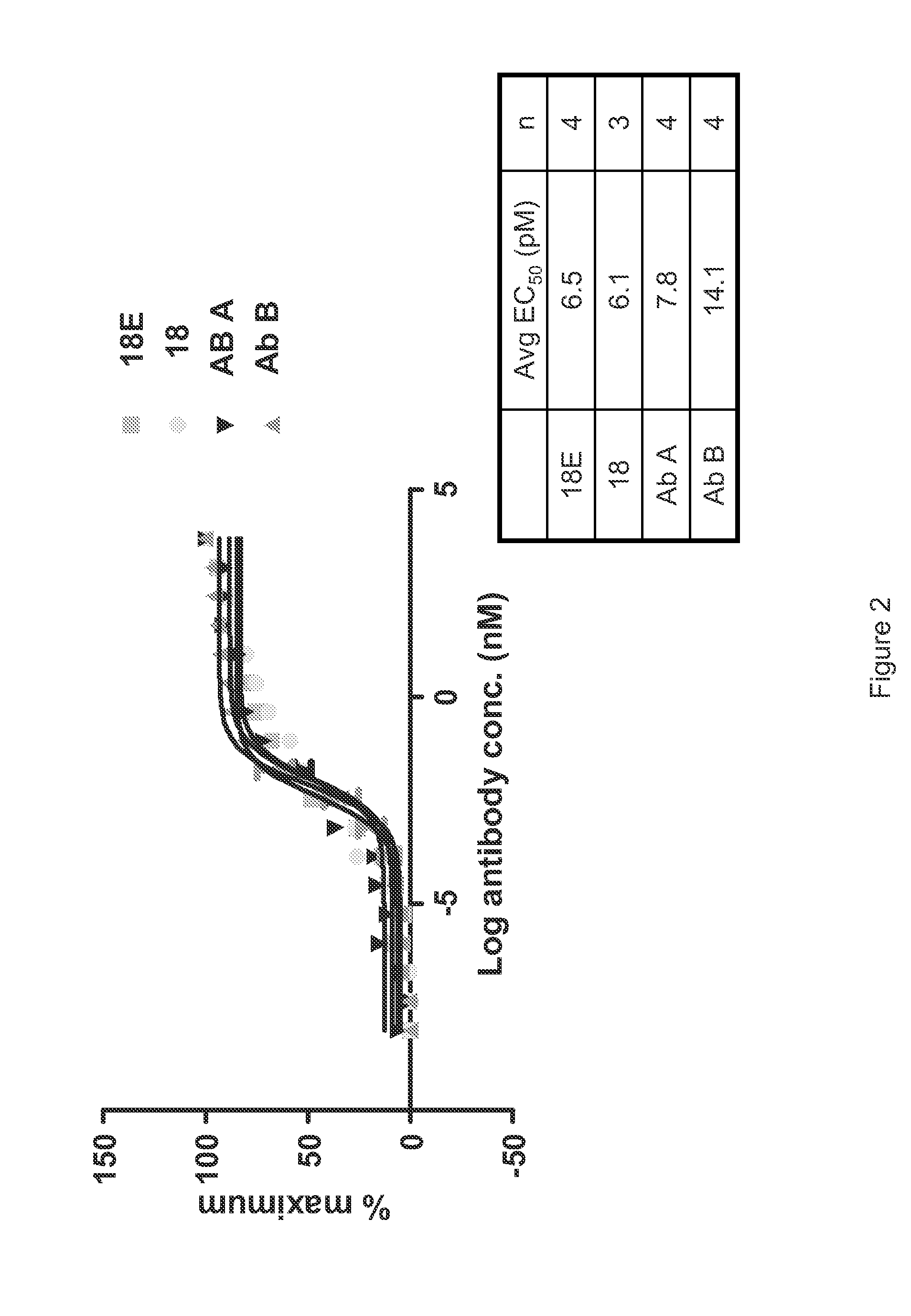
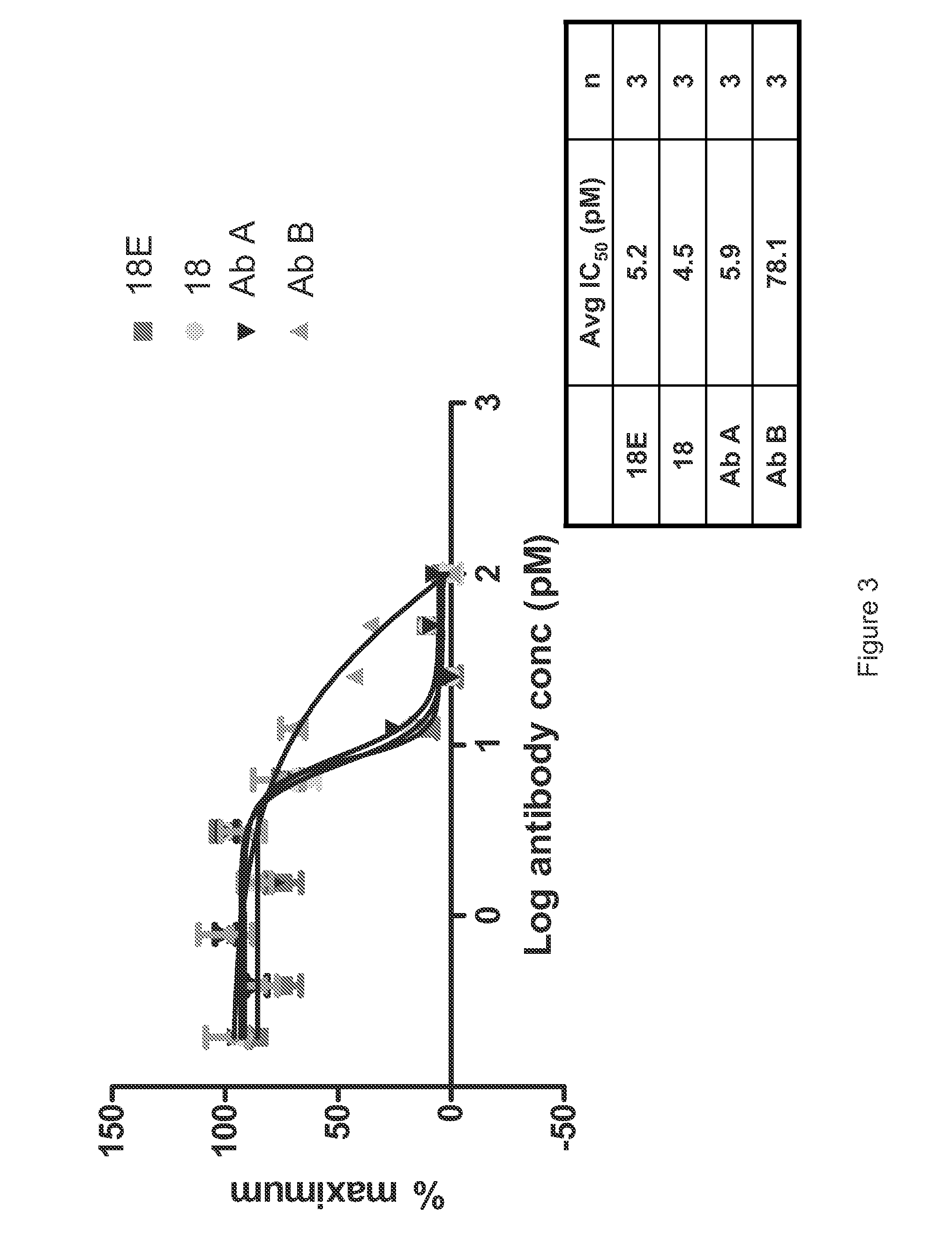
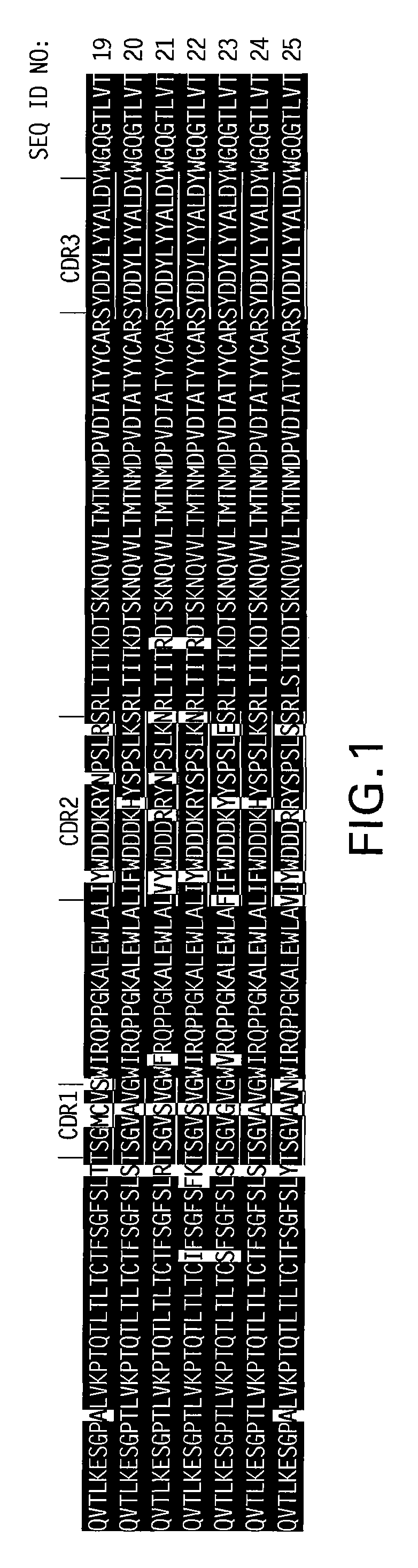
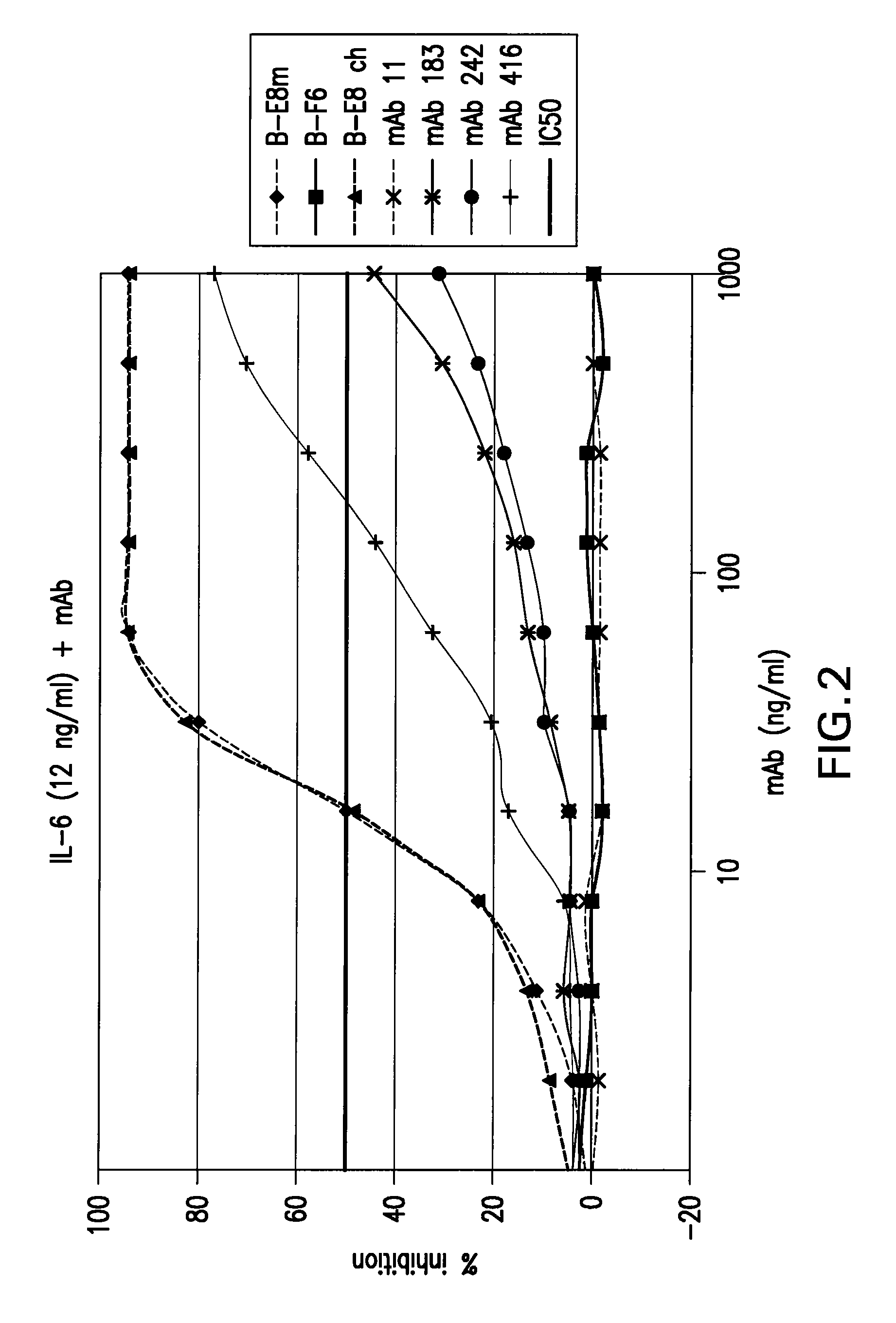
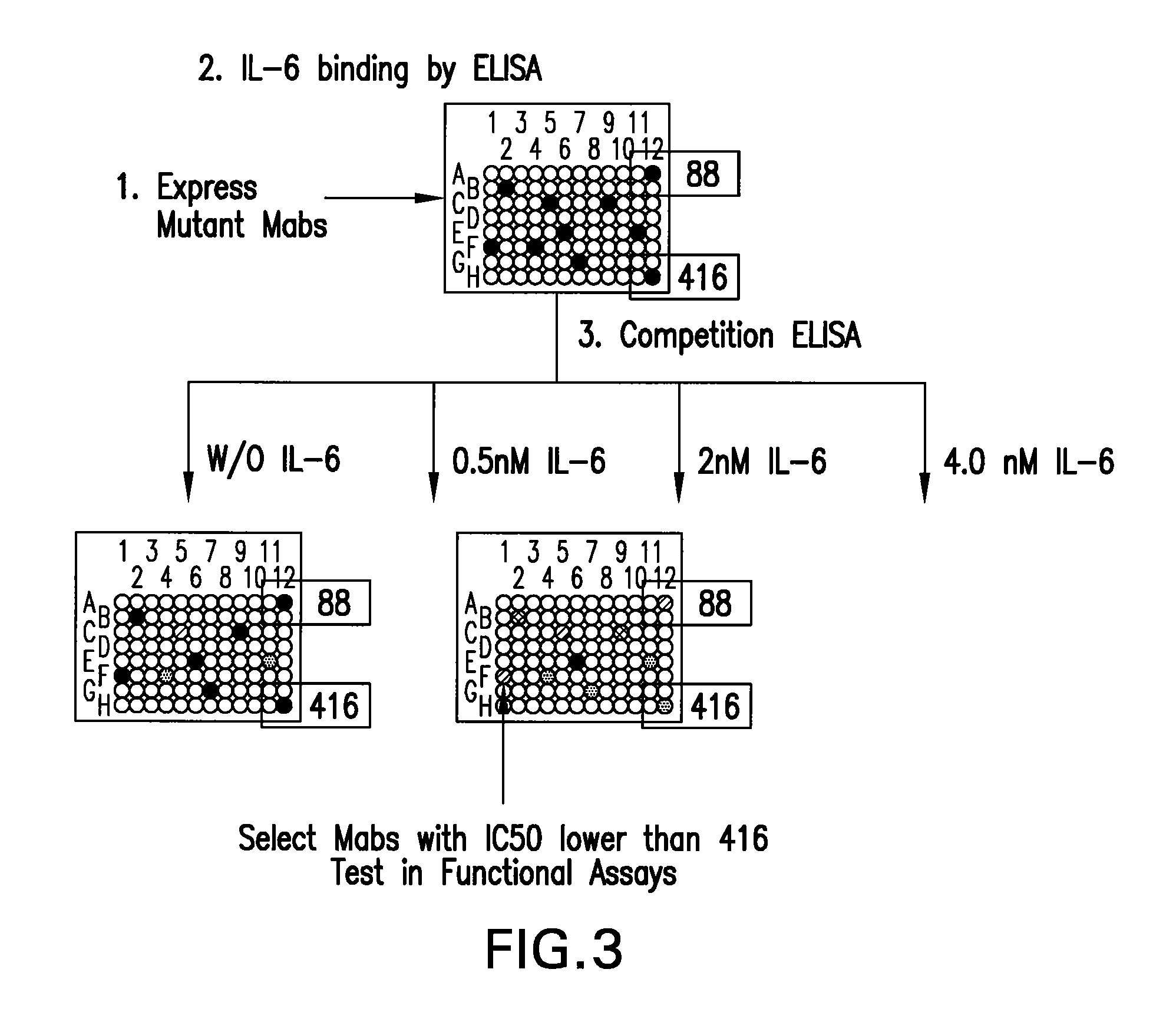
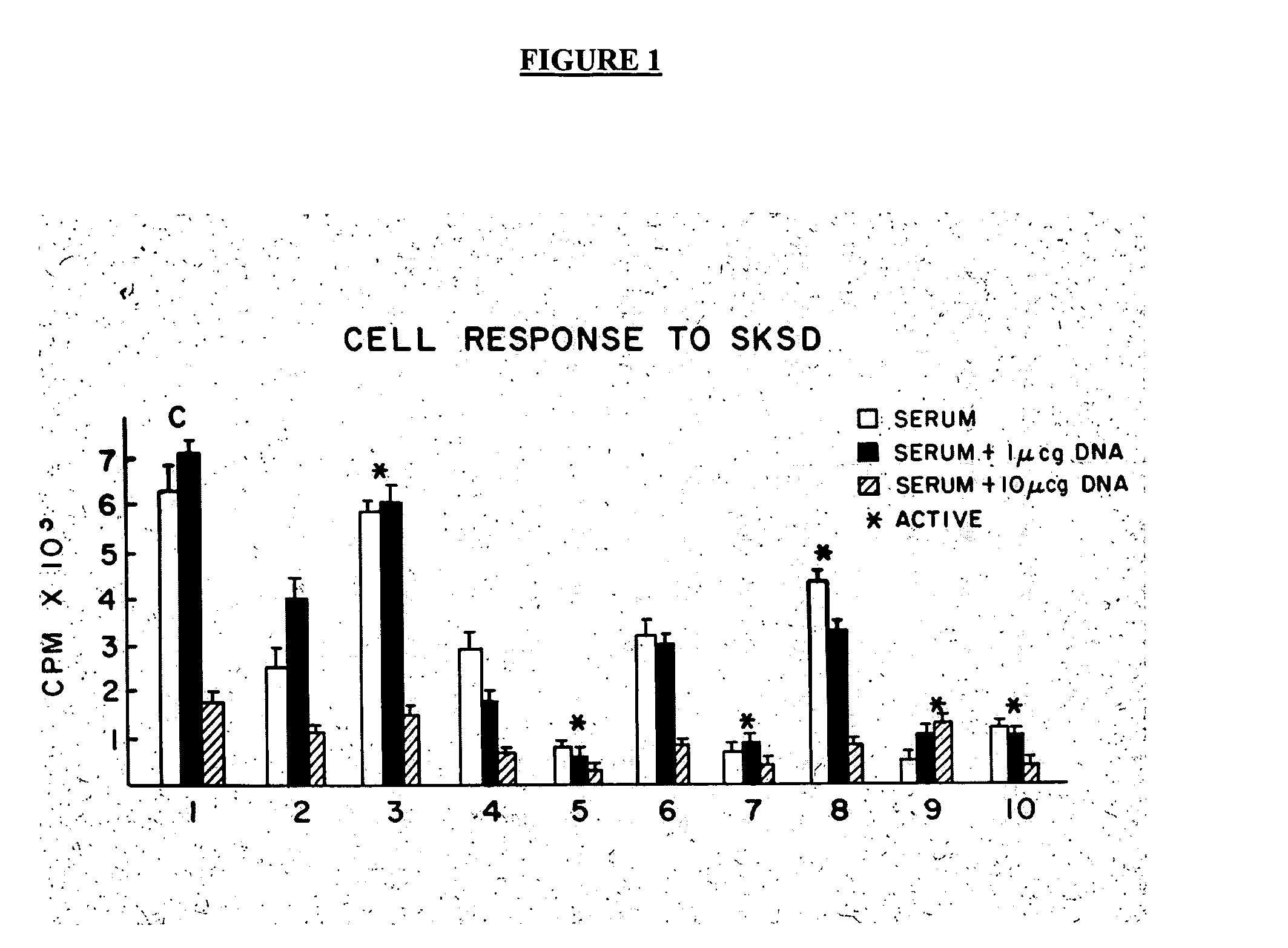
Patents
Literature
48 results about "Anti-IL-6" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
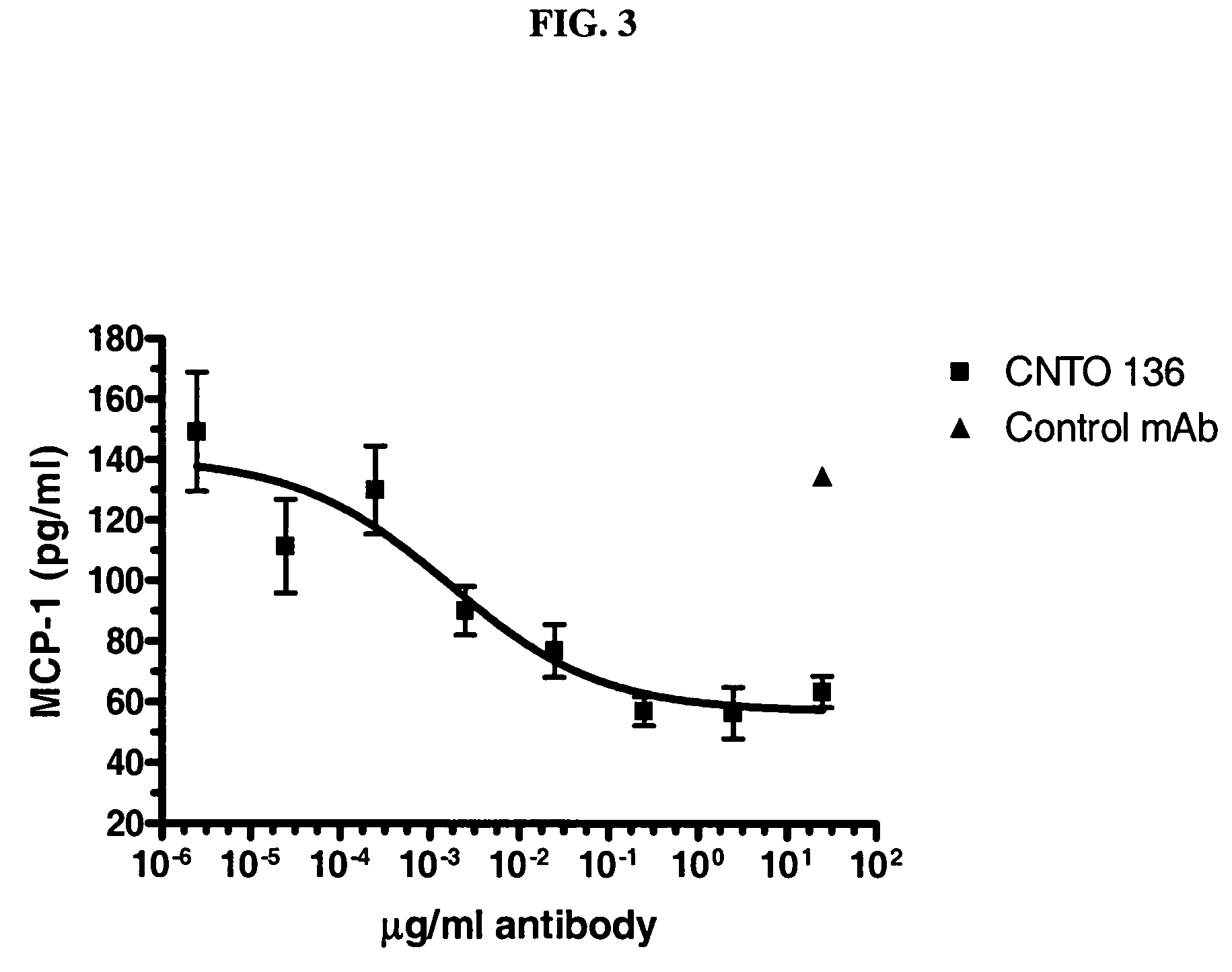
Anti-interleukin-6 agents are a class of therapeutics. Interleukin 6 is a cytokine relevant to many inflammatory diseases and many cancers. Hence, anti-IL6 agents have been sought. In rheumatoid arthritis they can help patients unresponsive to TNF inhibitors.
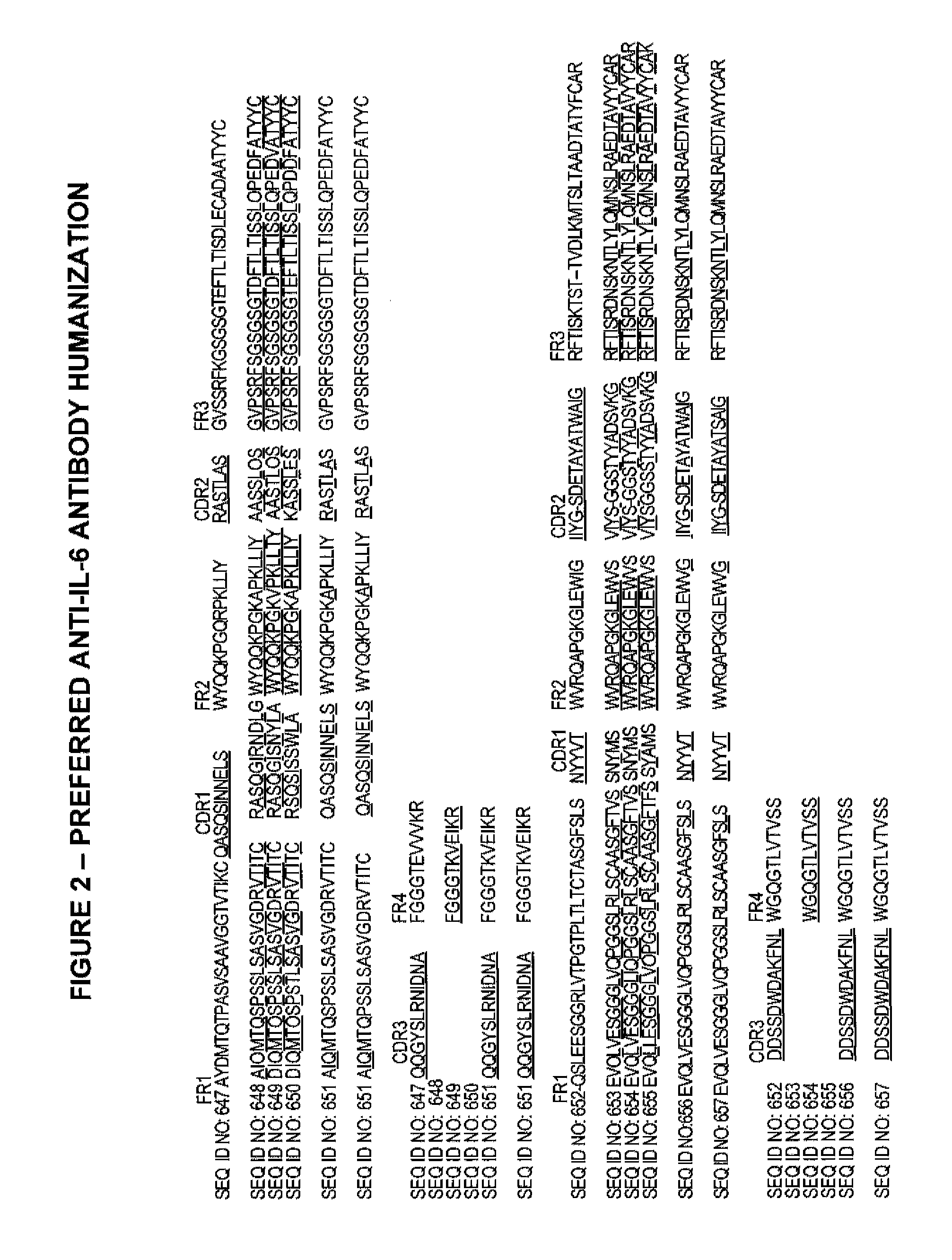
Anti-IL-6 antibodies, compositions, methods and uses
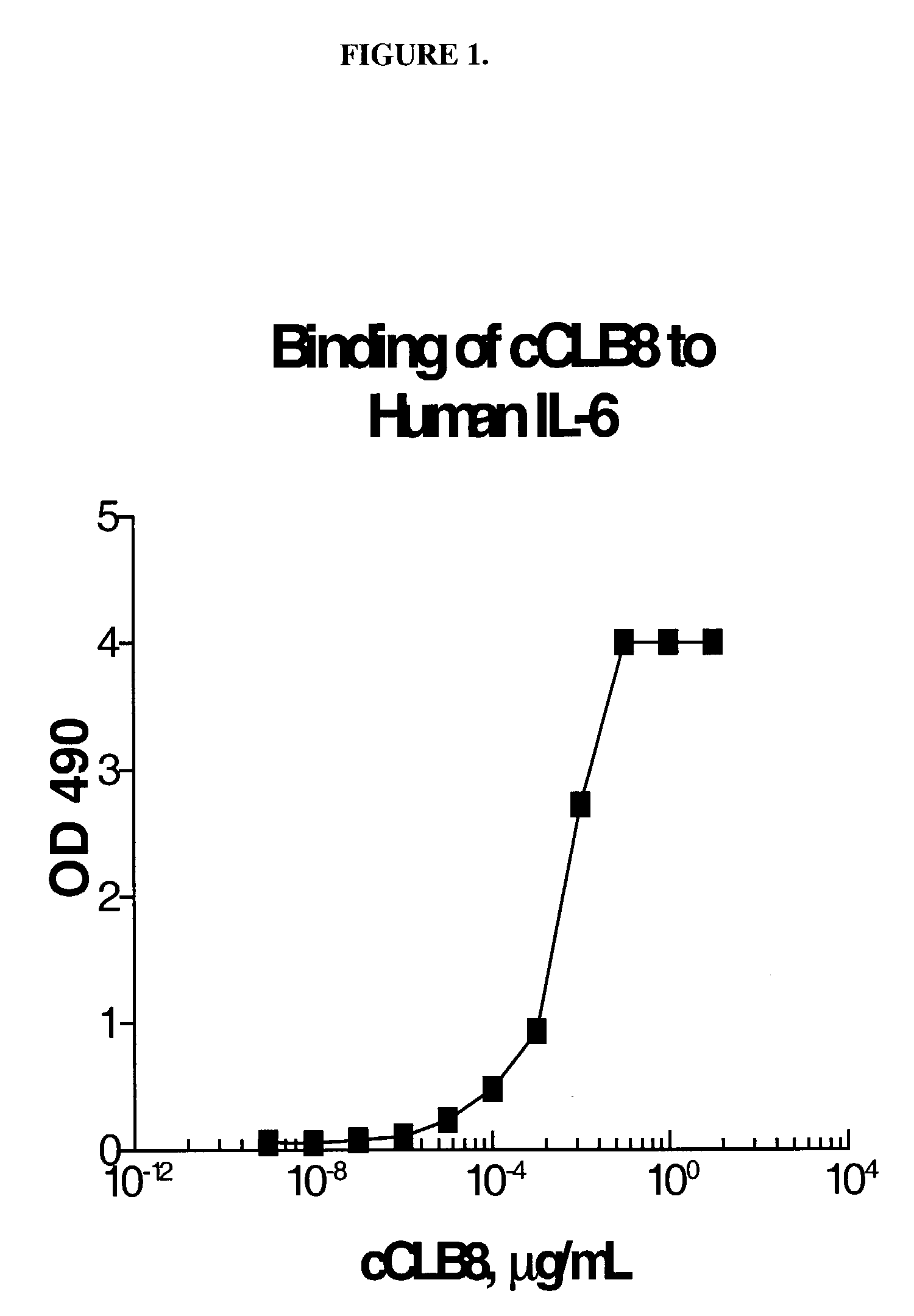
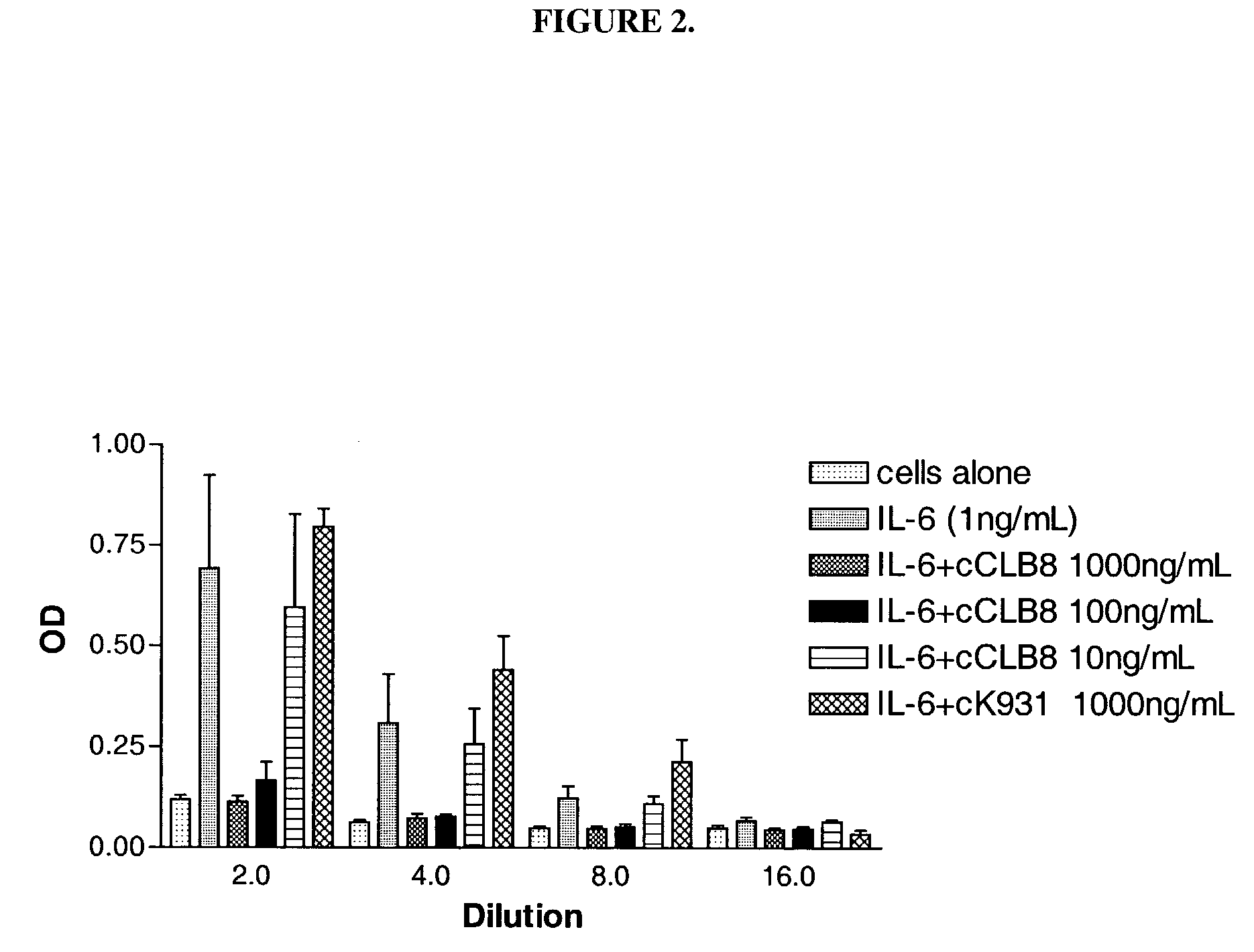
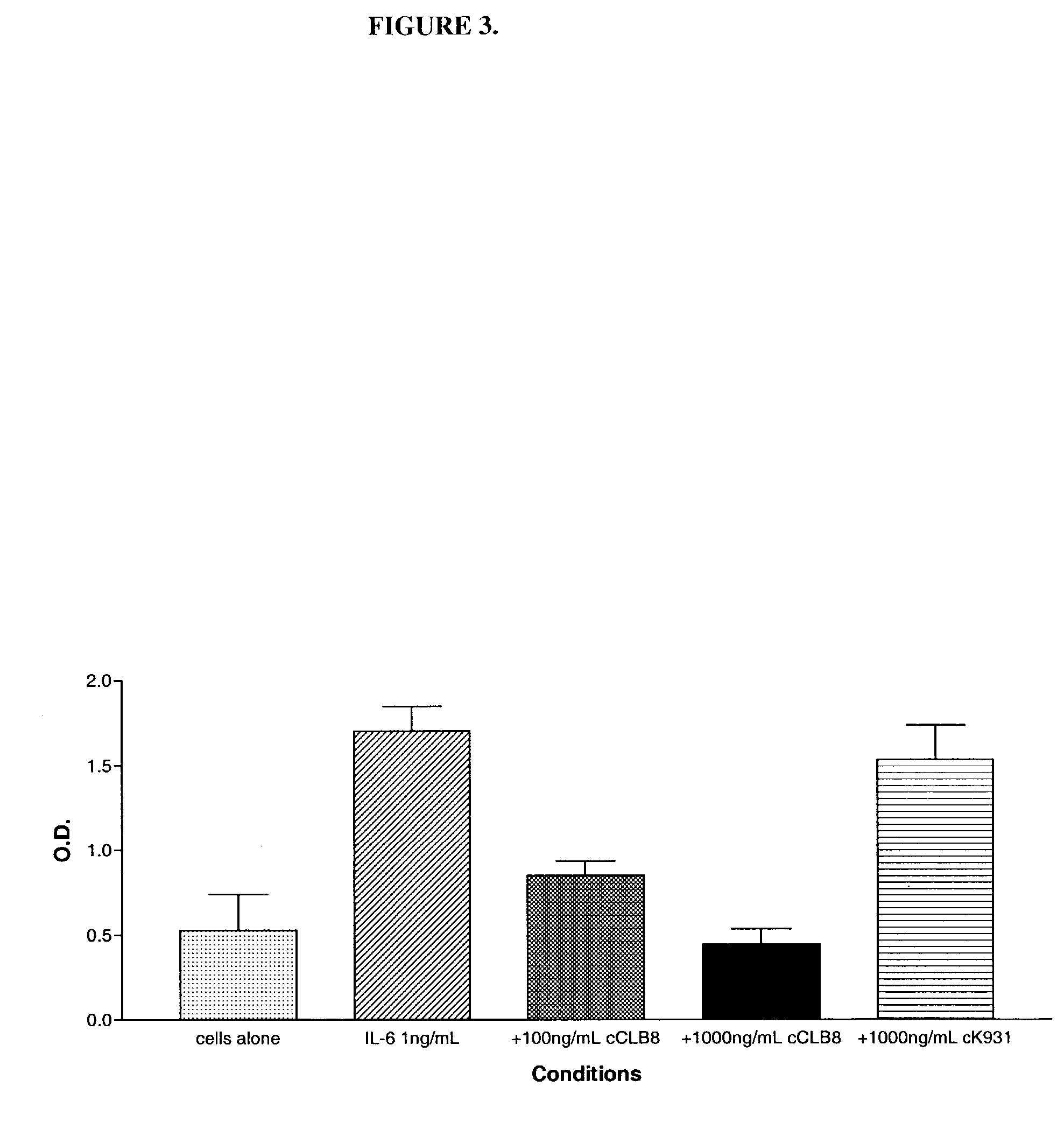
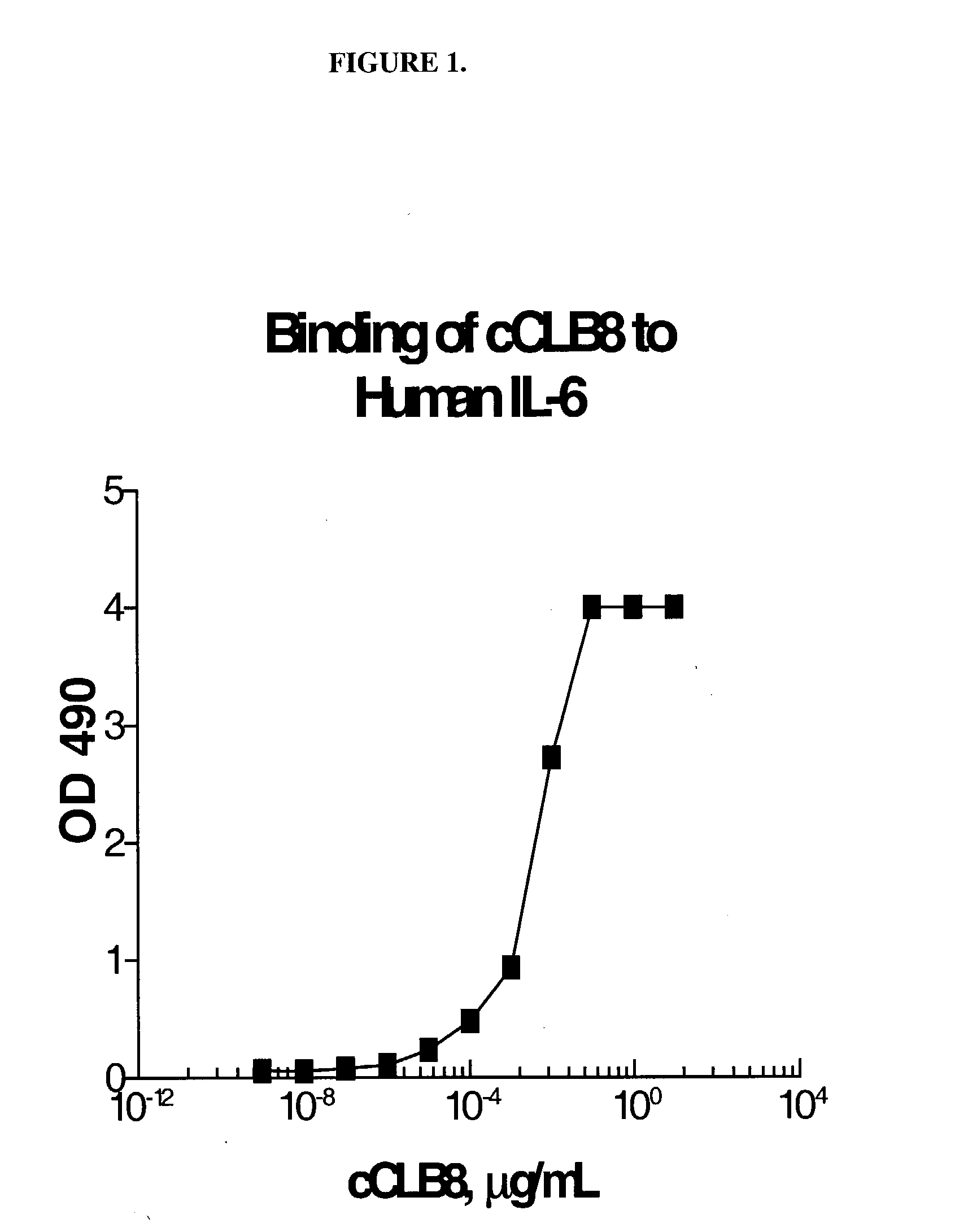
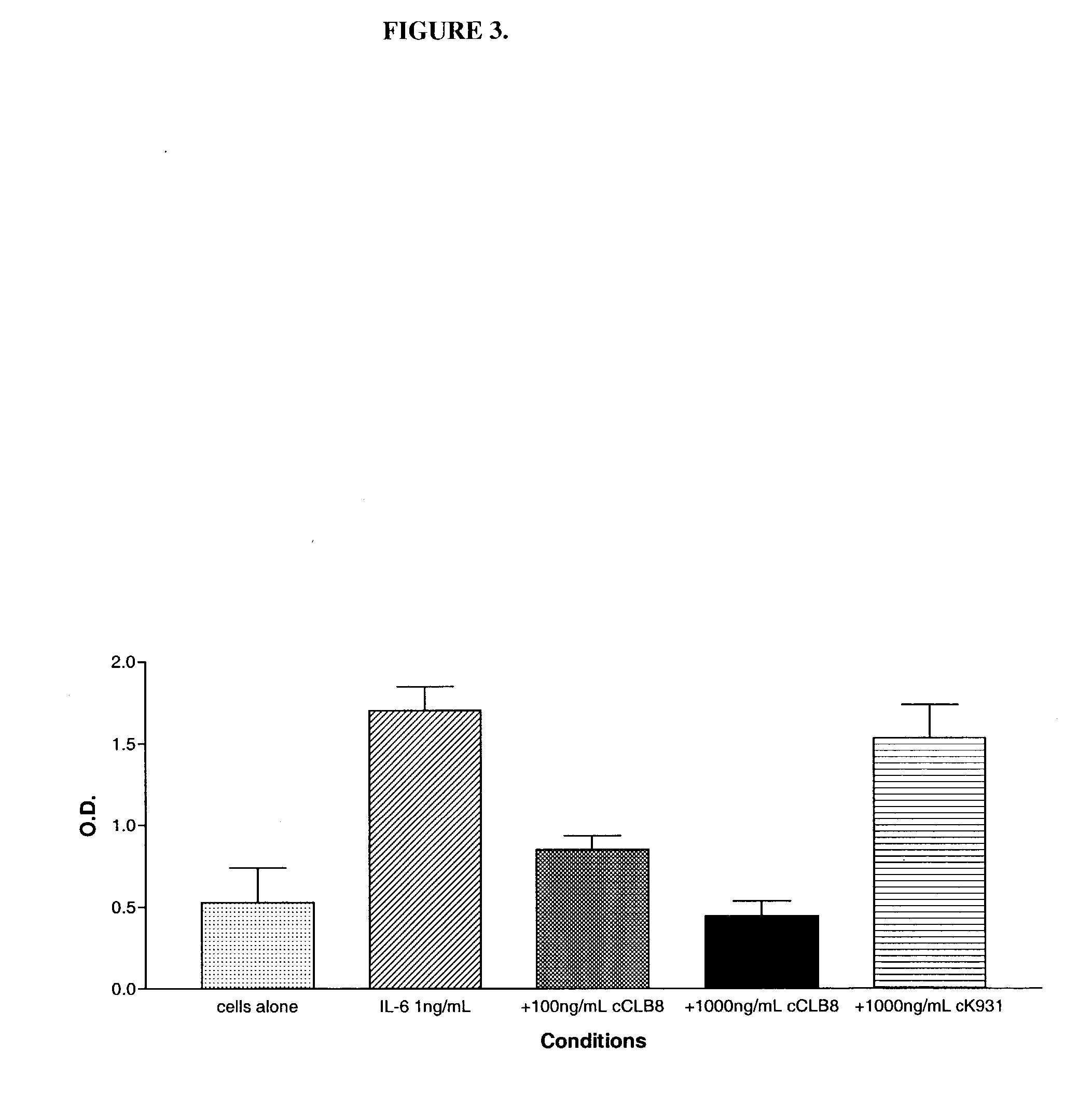
The present invention relates to at least one novel chimeric, humanized or CDR-grafted anti-IL-6 antibodies derived from the murine CLB-8 antibody, including isolated nucleic acids that encode at least one such anti-IL-6 antibody, vectors, host cells, transgenic animals or plants, and methods of making and using thereof, including therapeutic compositions, methods and devices.
Owner:CENTOCOR
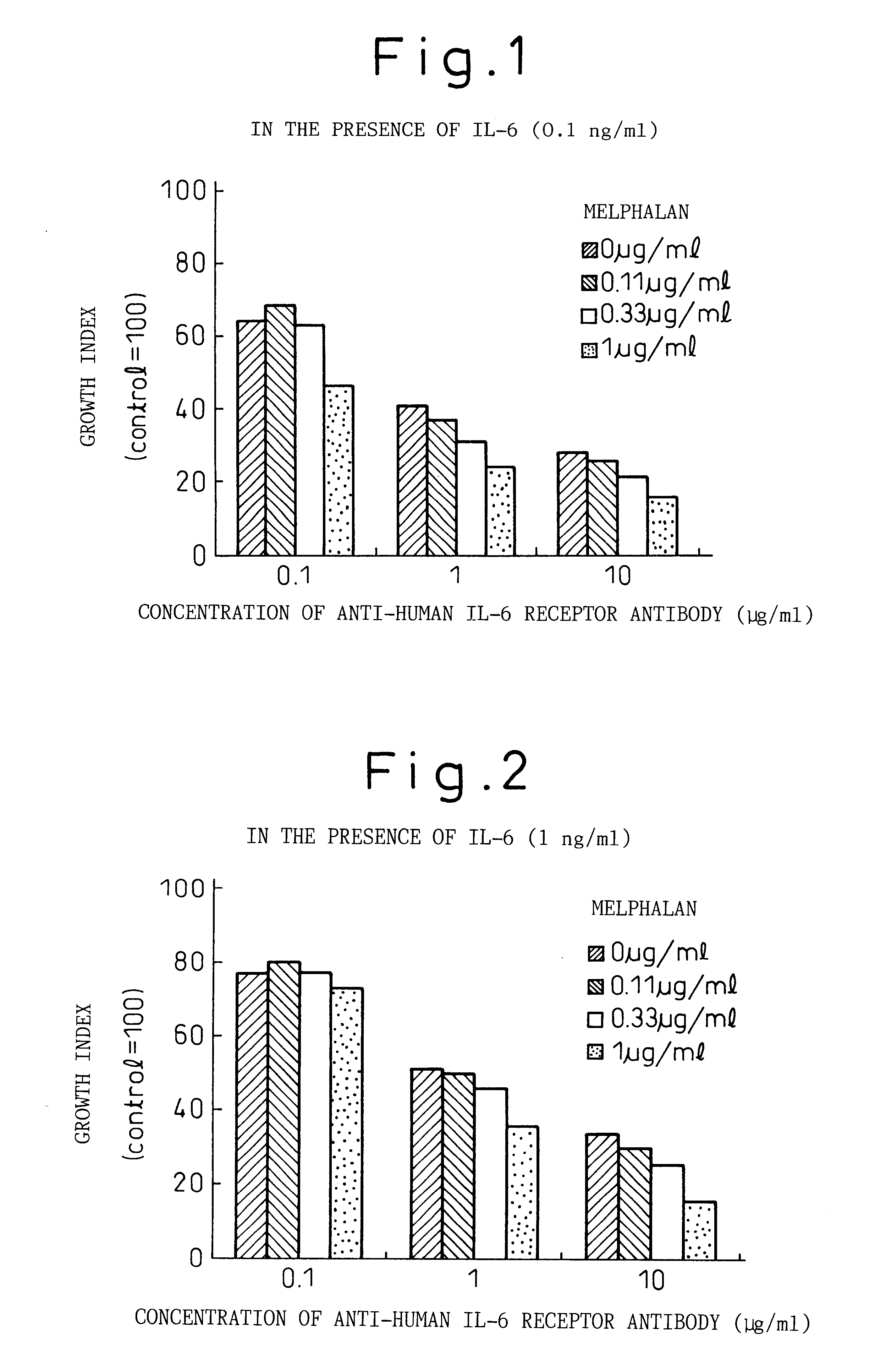
Anti-IL-6 Receptor Antibody
InactiveUS20110245473A1Enhanced antigen-neutralizing activity and pharmacokineticsGood treatment effectCompound screeningApoptosis detectionHigh concentrationHinge region
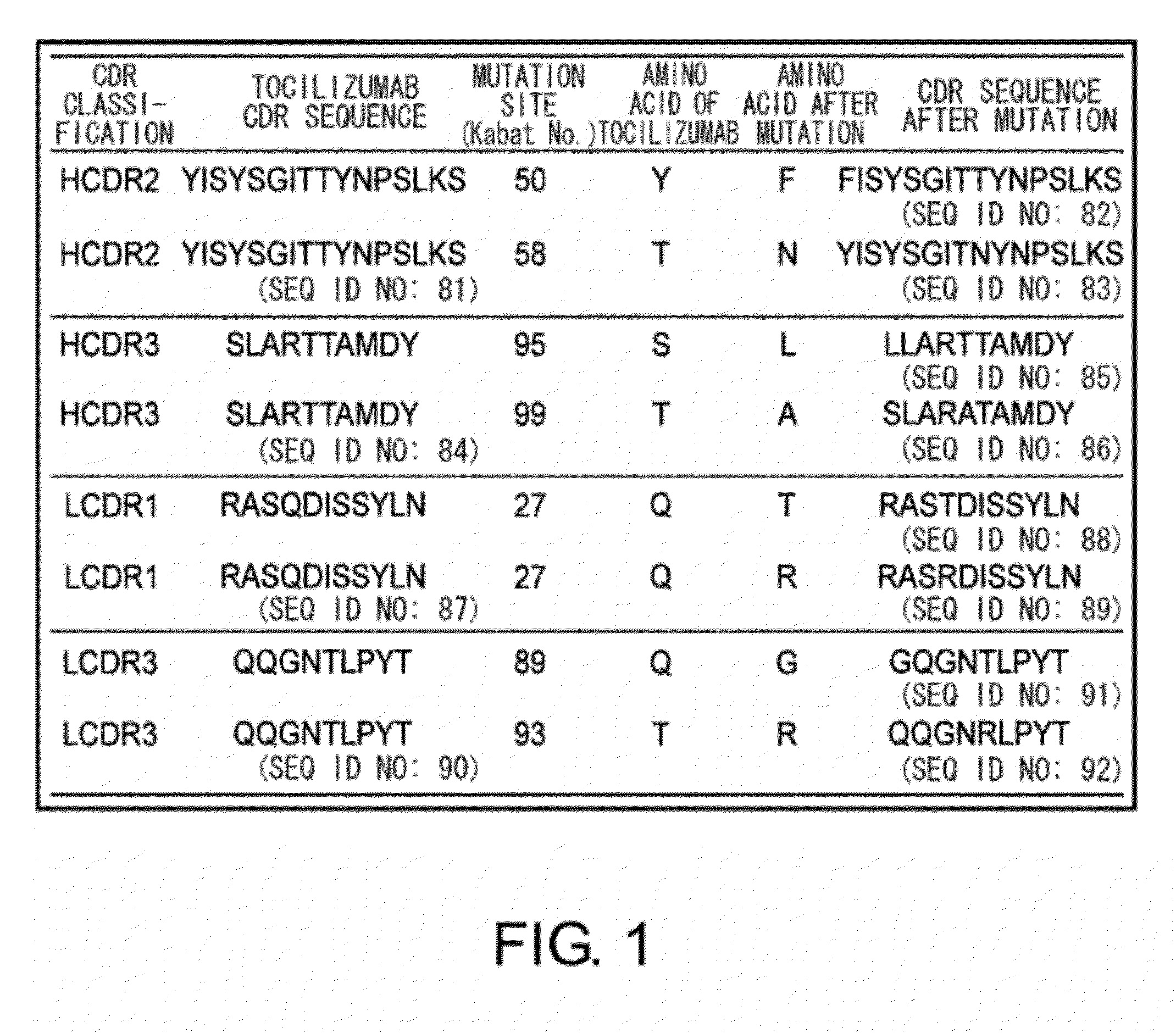
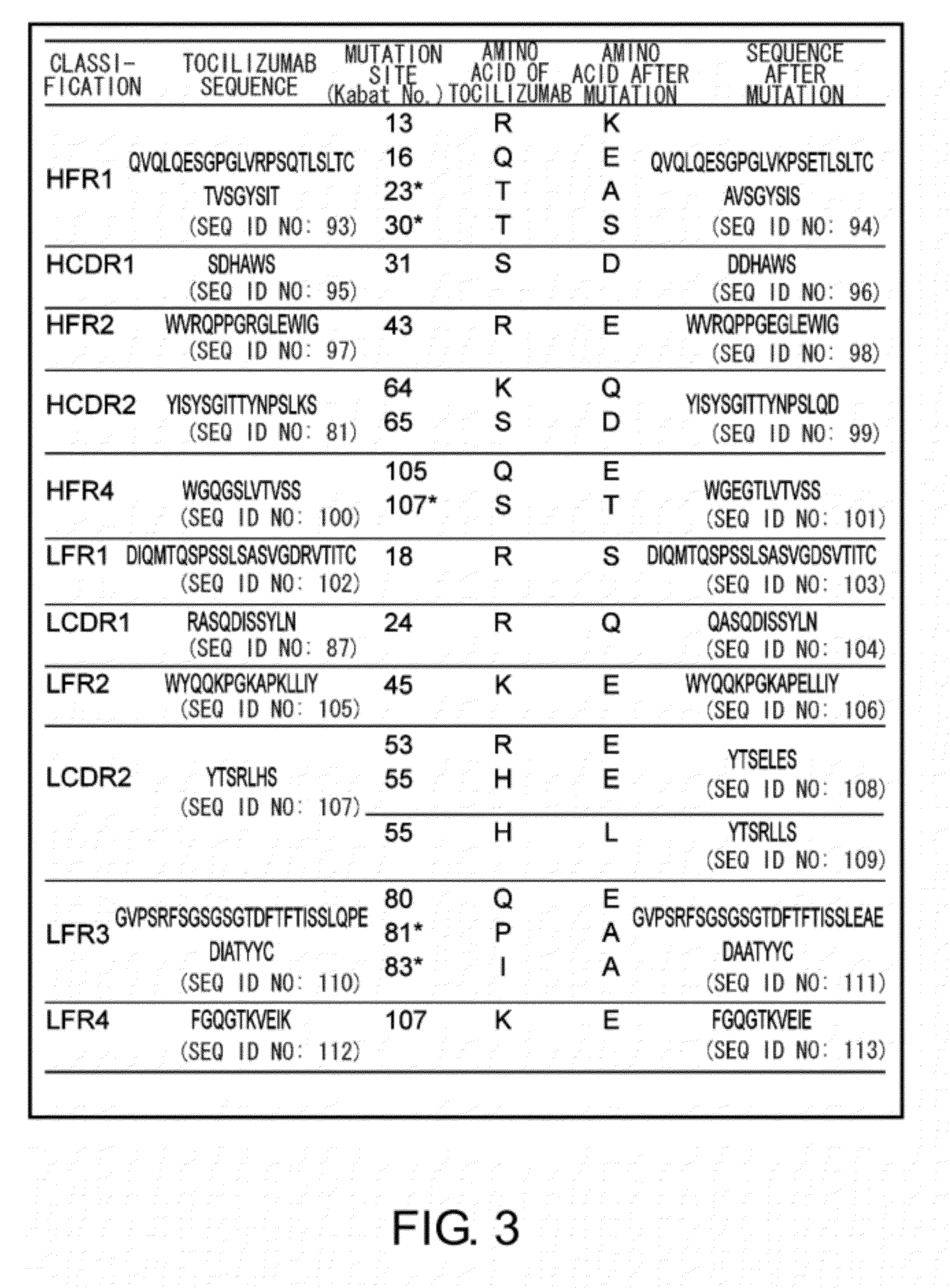
The present inventors succeeded in discovering specific amino acid mutations in the variable region, framework region, and constant region of TOCILIZUMAB, and this enables to reduce immunogenicity risk and the heterogeneity originated from disulfide bonds in the hinge region, as well as to improve antigen binding activity, pharmacokinetics, stability under acidic conditions, and stability in high concentration preparations.
Owner:CHUGAI PHARMA CO LTD
Anti-IL-6 antibodies, compositions, methods and uses
ActiveUS20060257407A1Avoid developmentEasy to controlAntibacterial agentsSenses disorderAntibodyAnti-IL-6
Owner:ORTHO BIOTECH
Preventive and/or therapeutic method for systemic lupus erythematosus comprising anti-IL-6 receptor antibody administration
InactiveUS20090022719A1Peptide/protein ingredientsAntibody mimetics/scaffoldsBULK ACTIVE INGREDIENTActive ingredient
A preventive and / or therapeutic agent for systemic lupus erythematosus comprising an anti-interleukin-6 (IL-6) receptor antibody as an active ingredient.
Owner:CHUGAI PHARMA CO LTD
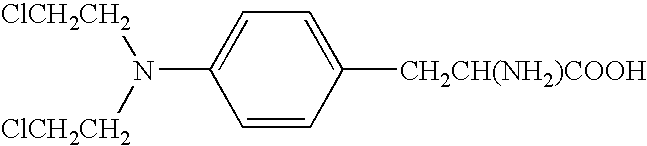
Remedies for myeloma to be used together with nitrogen mustard antitumor agents
A therapeutic agent for myeloma comprising a combined use of a nitrogen mustard anticancer agent and anti-IL-6 receptor antibody. Thus, a therapeutic agent for myeloma comprising anti-IL-6 receptor antibody for use in combination with a nitrogen mustard anticancer agent; a therapeutic agent for myeloma comprising a nitrogen mustard anticancer agent for use in combination with anti-IL-6 receptor antibody; and a therapeutic agent for myeloma comprising a nitrogen mustard anticancer agent and anti-IL-6 receptor antibody.
Owner:CHUGAI PHARMA CO LTD
Anti-il-6 antibodies, compositions, methods and uses
ActiveUS7560112B2Avoid developmentEasy to controlAntibacterial agentsSenses disorderAntibodyAnti-IL-6
Owner:ORTHO BIOTECH
Chronic Rejection Inhibitor
ActiveUS20100061986A1Suppressing chronic rejection reactionAvoid spreadingAntibody ingredientsImmunoglobulinsHeart transplantationStenotic lesion
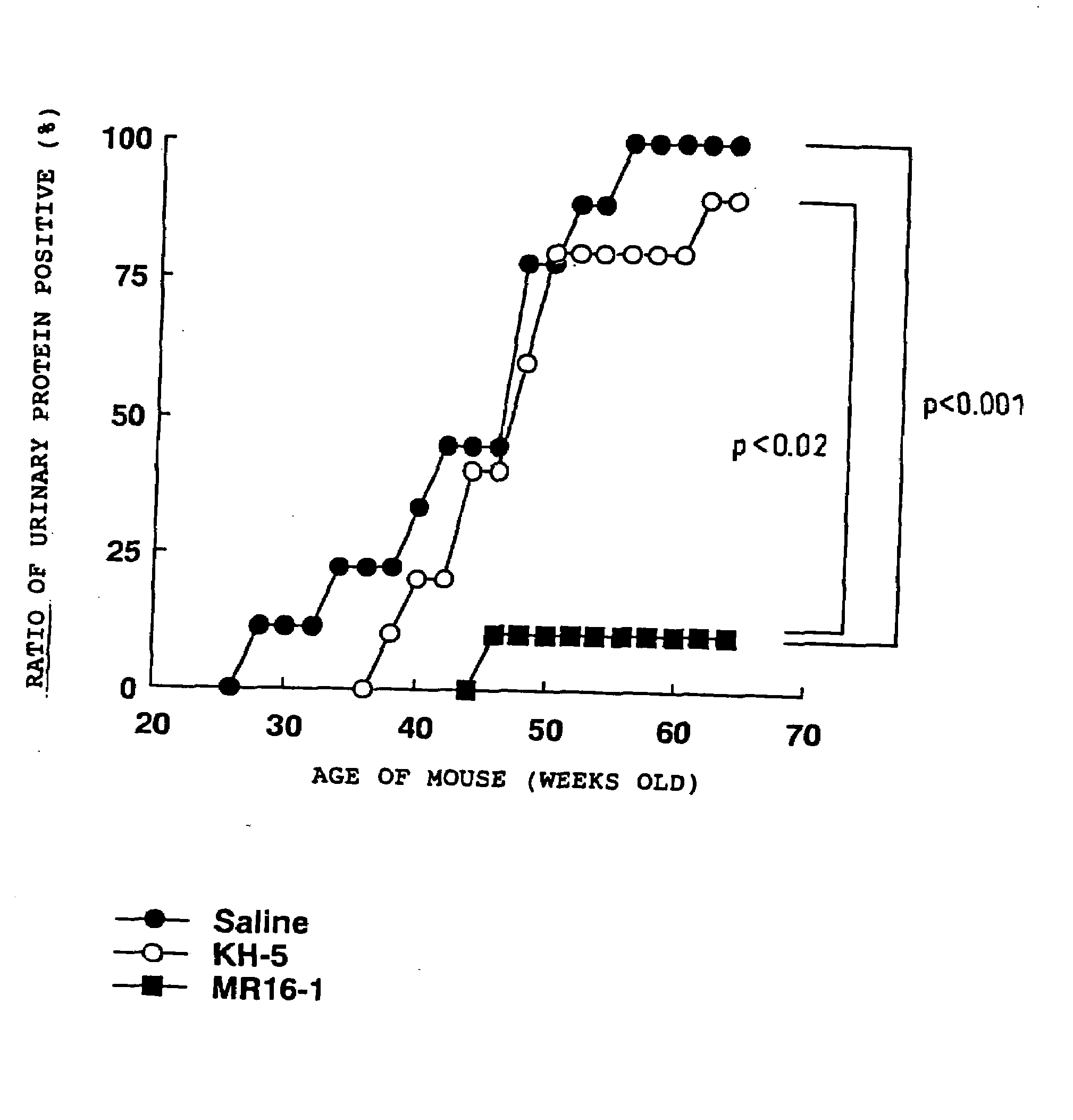
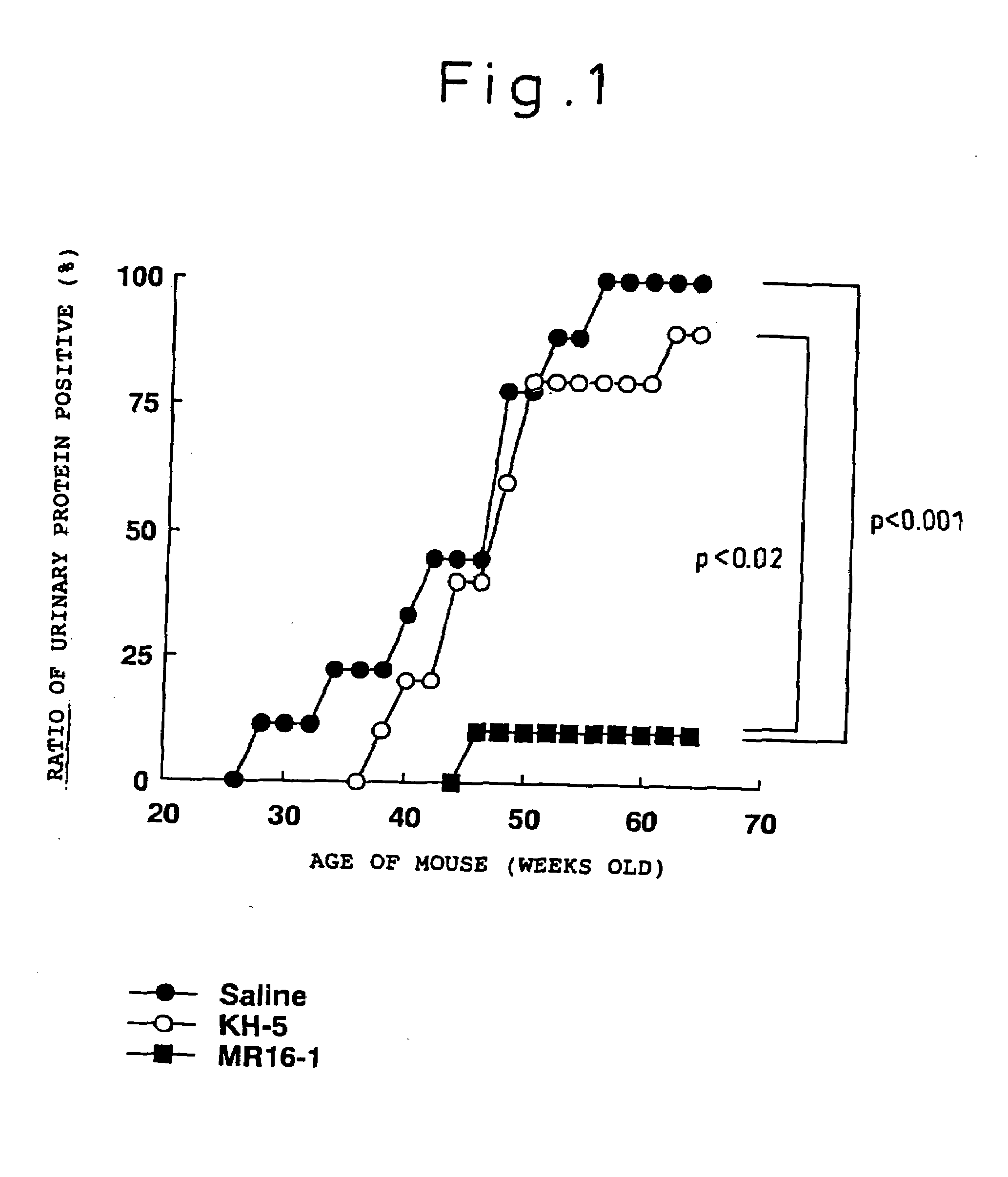
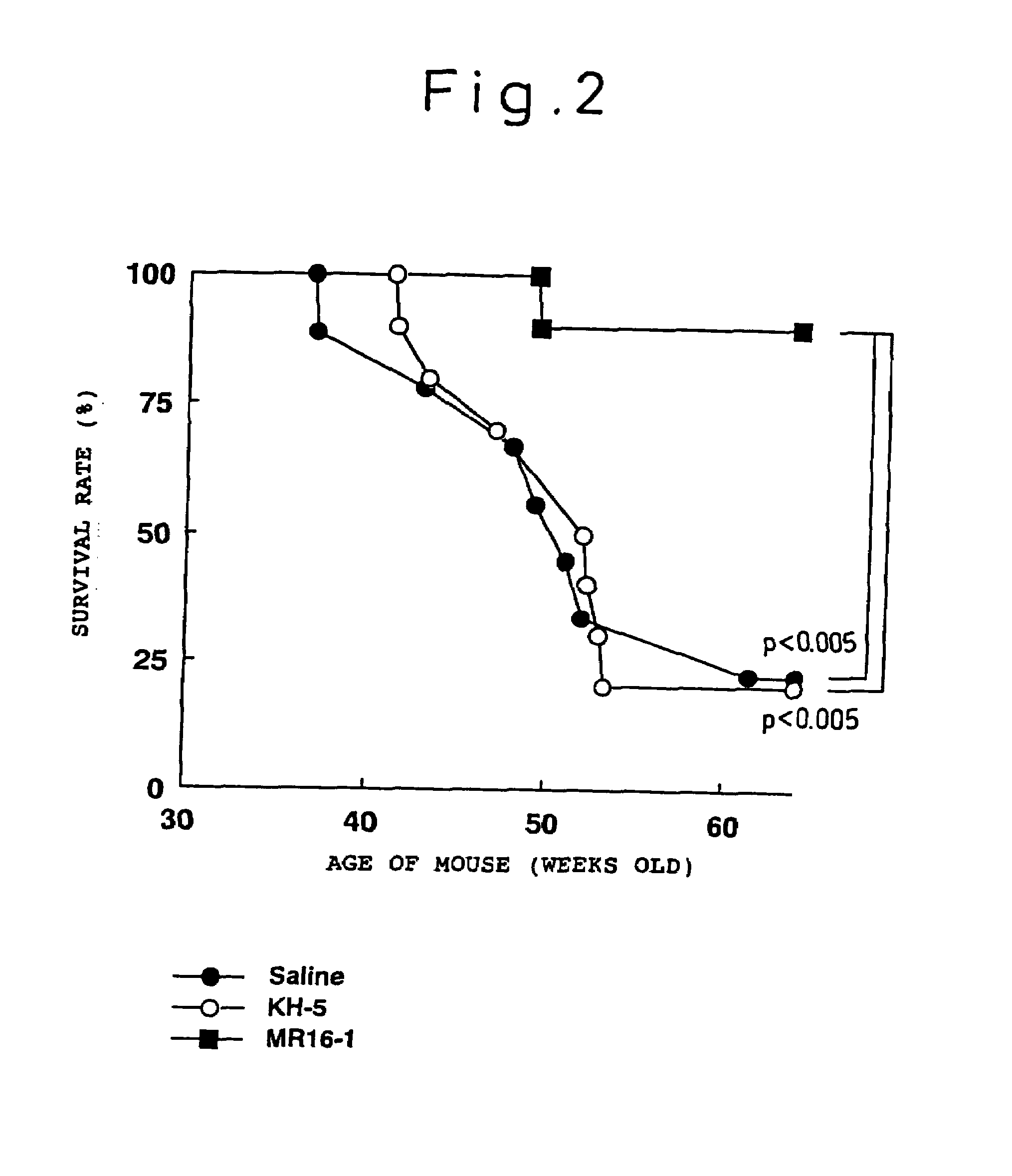
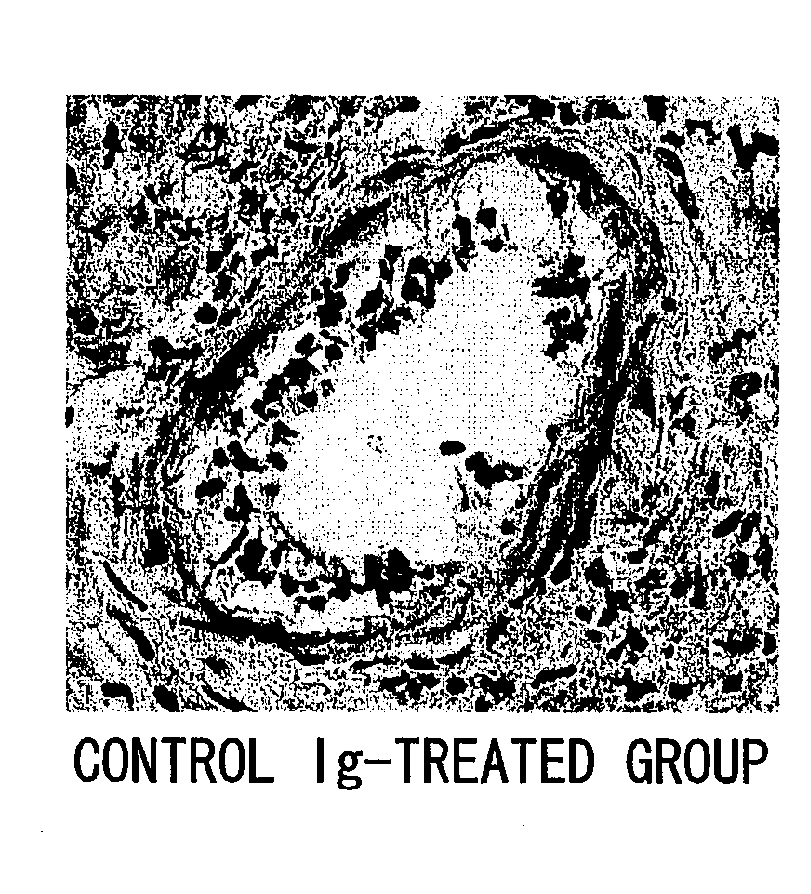
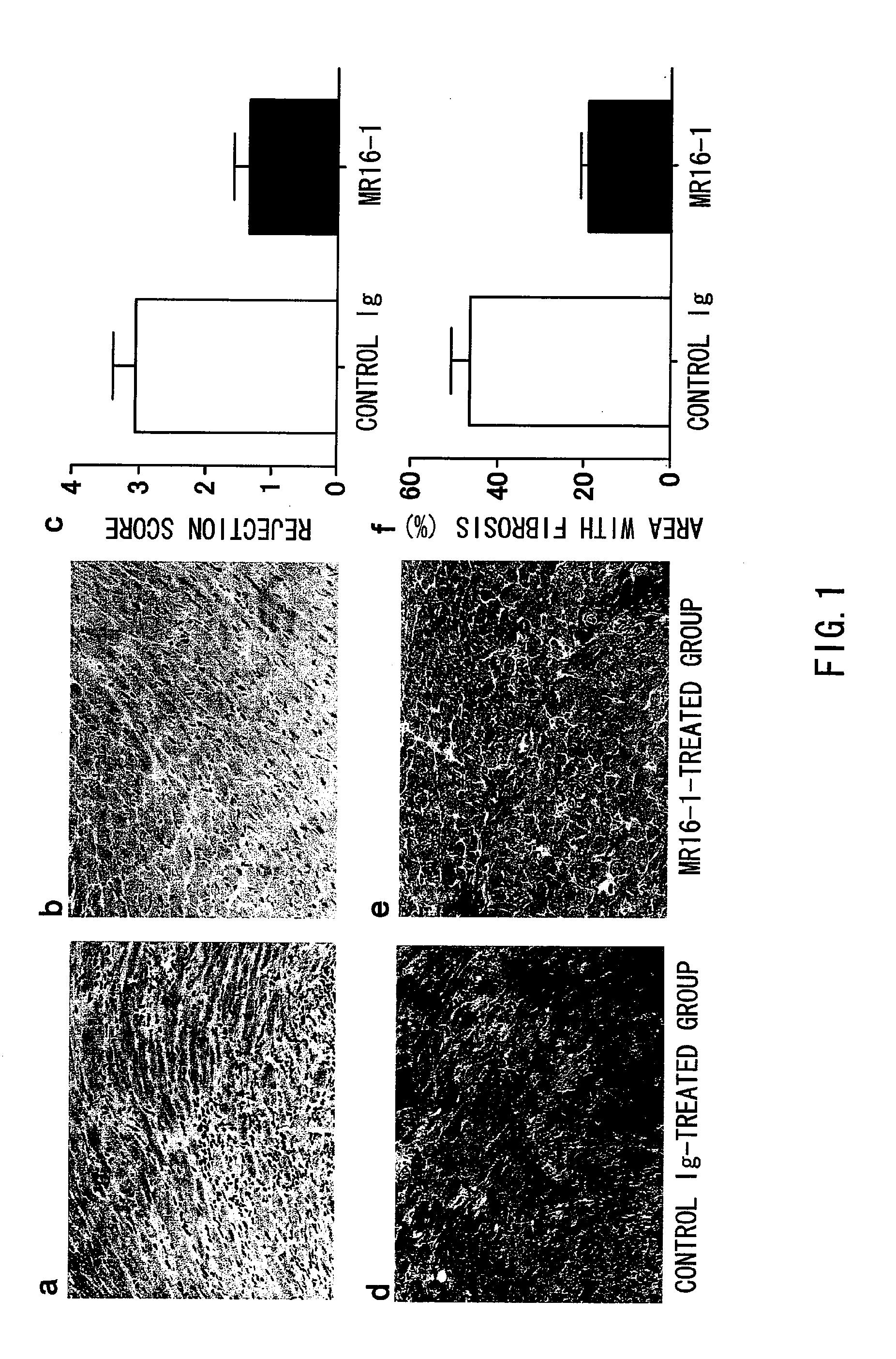
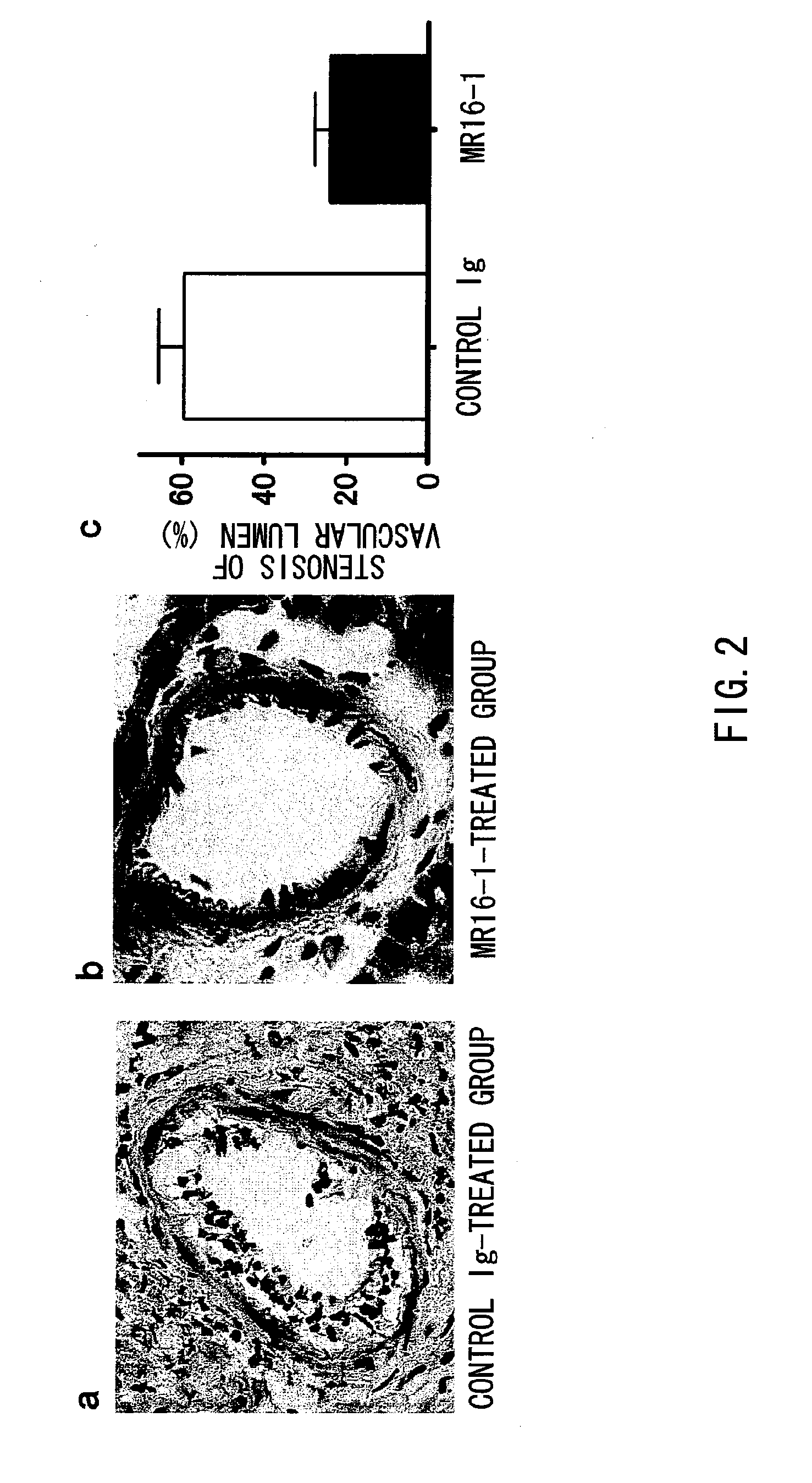
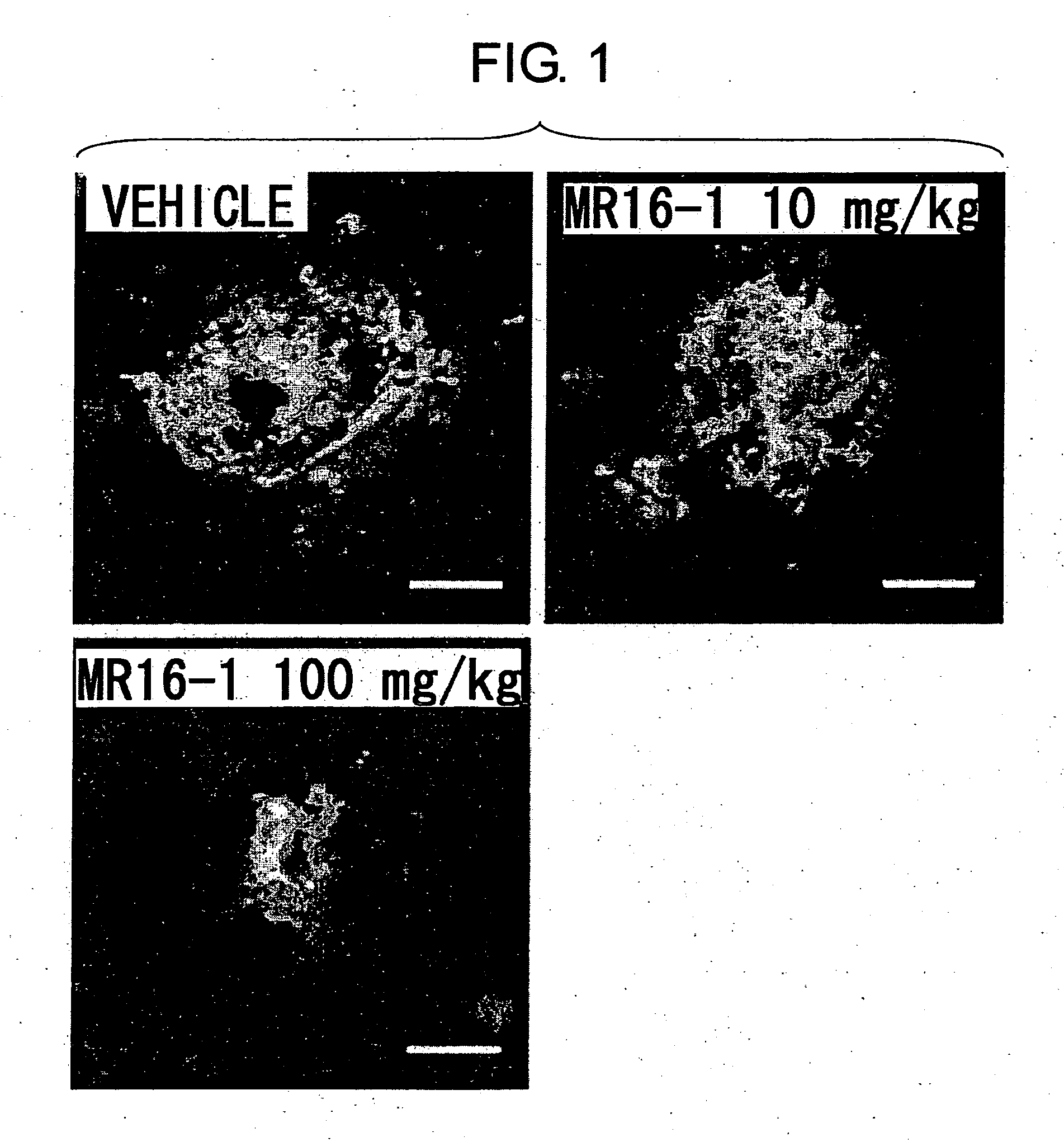
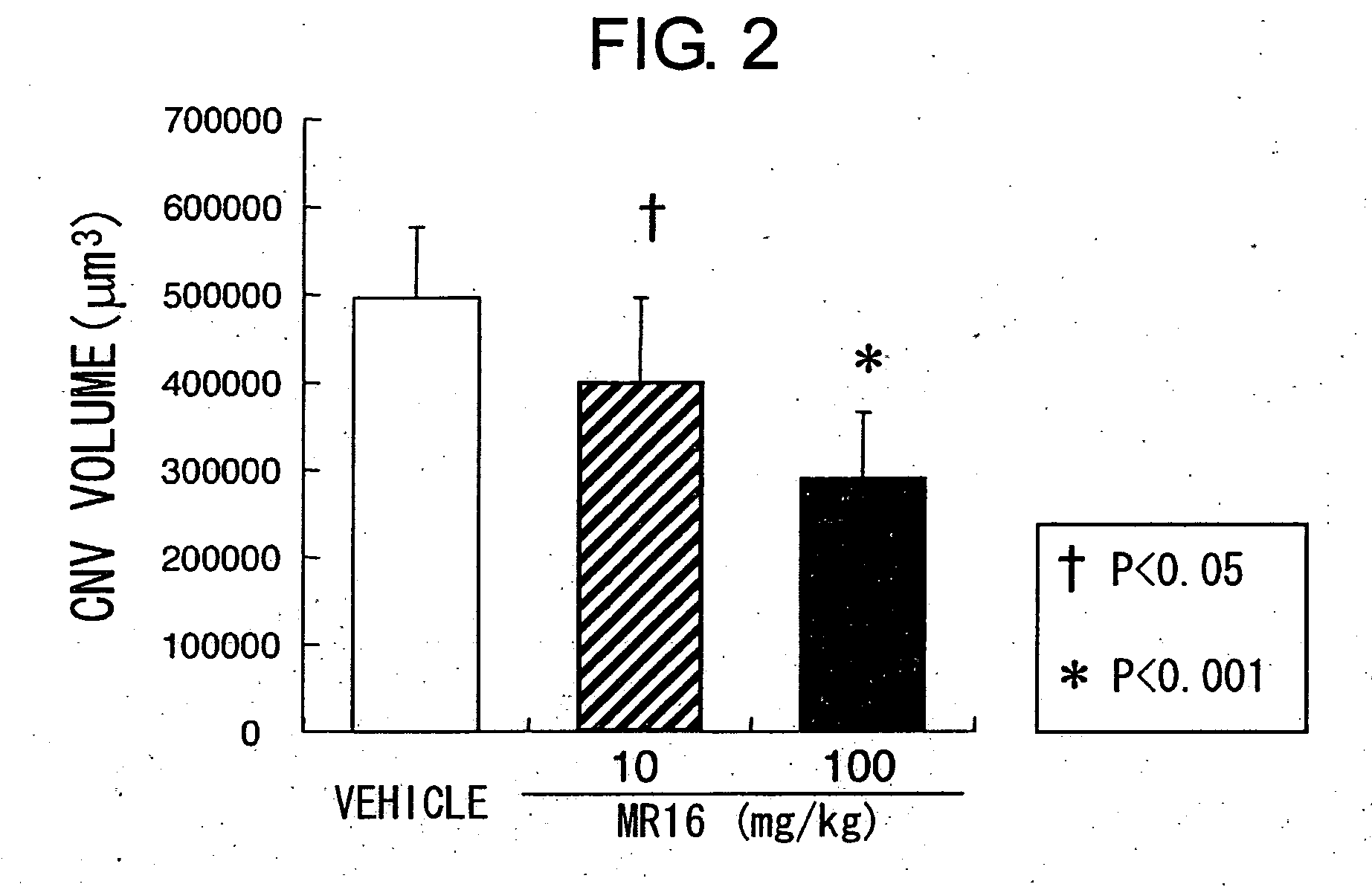
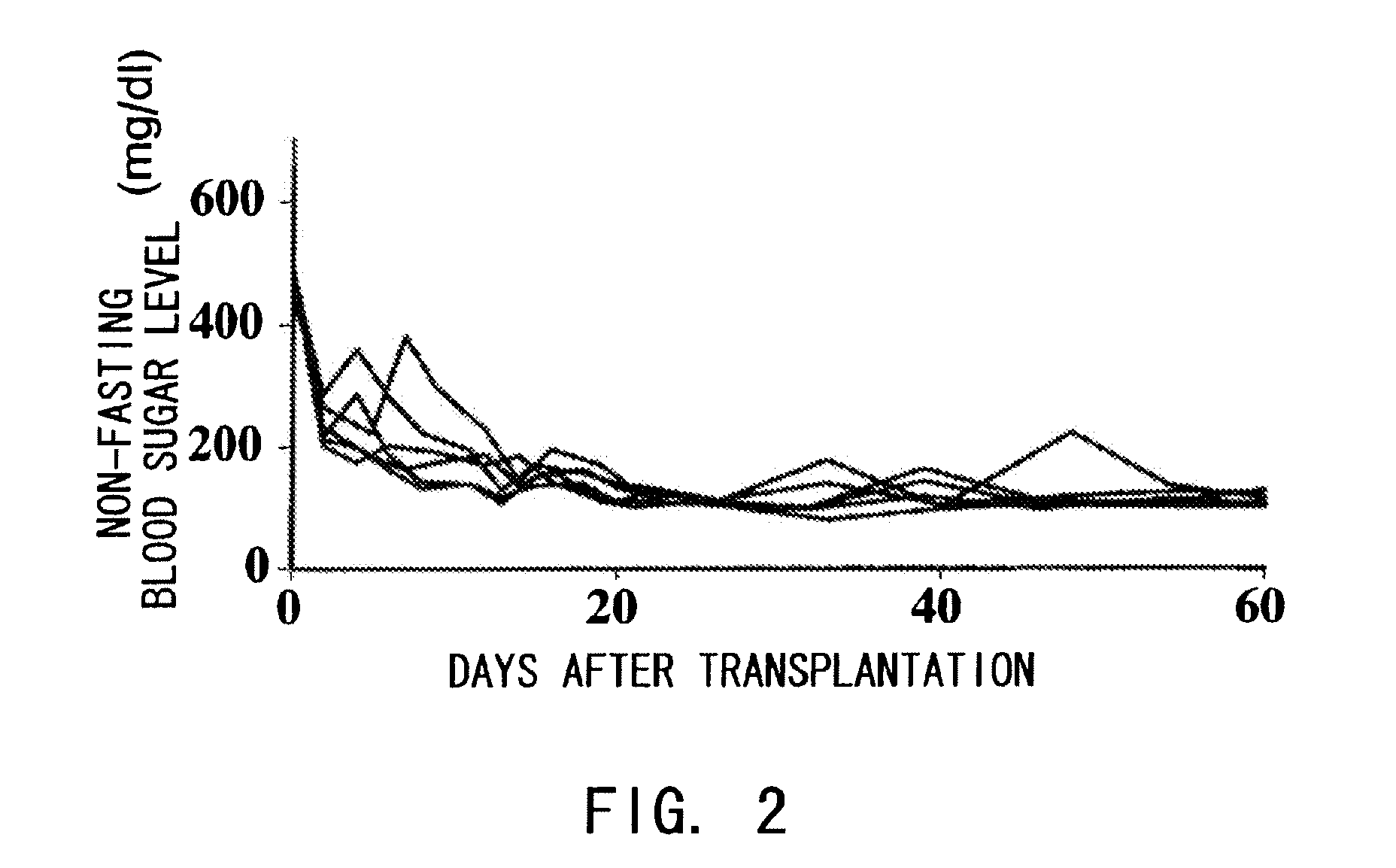
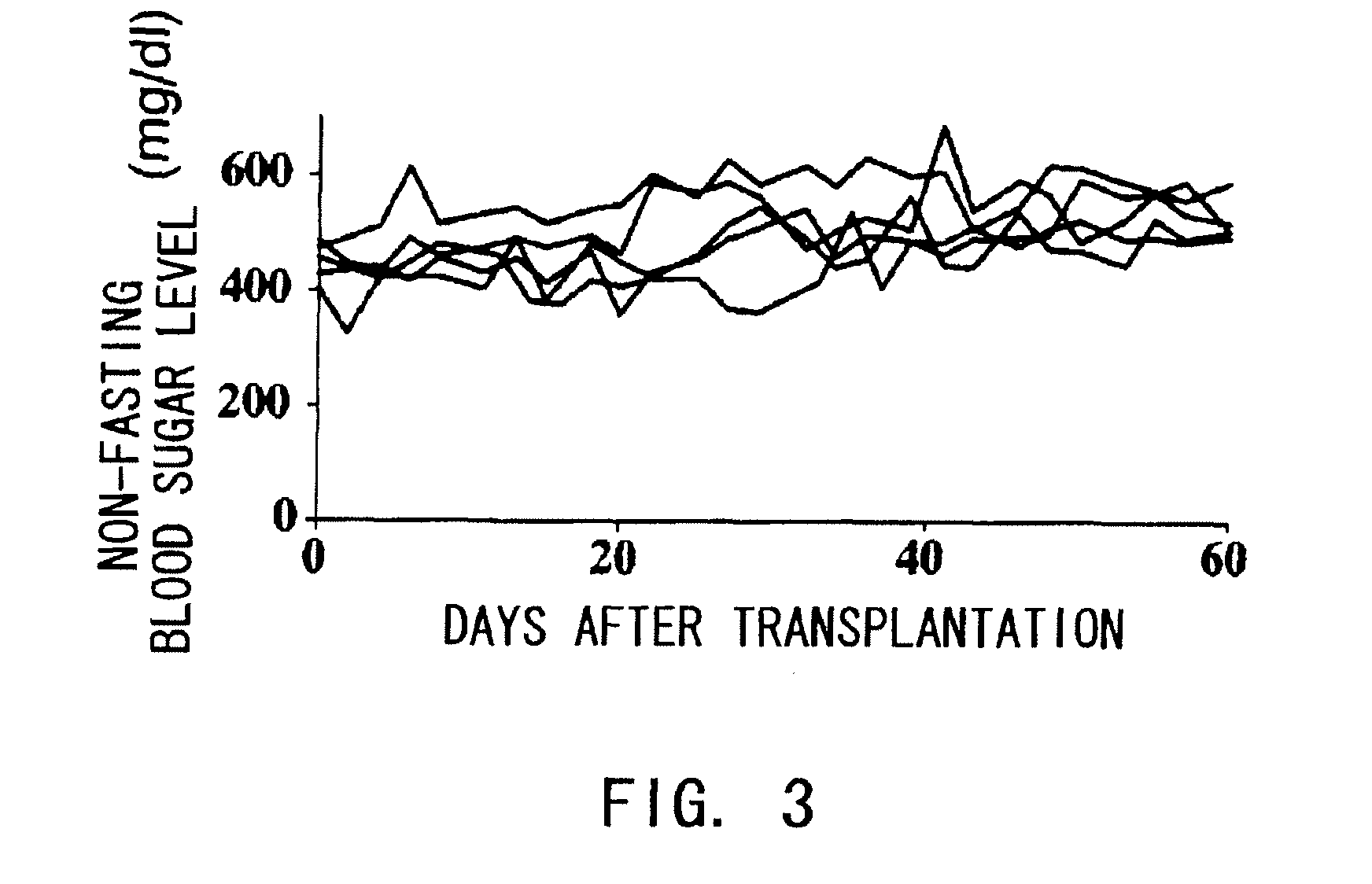
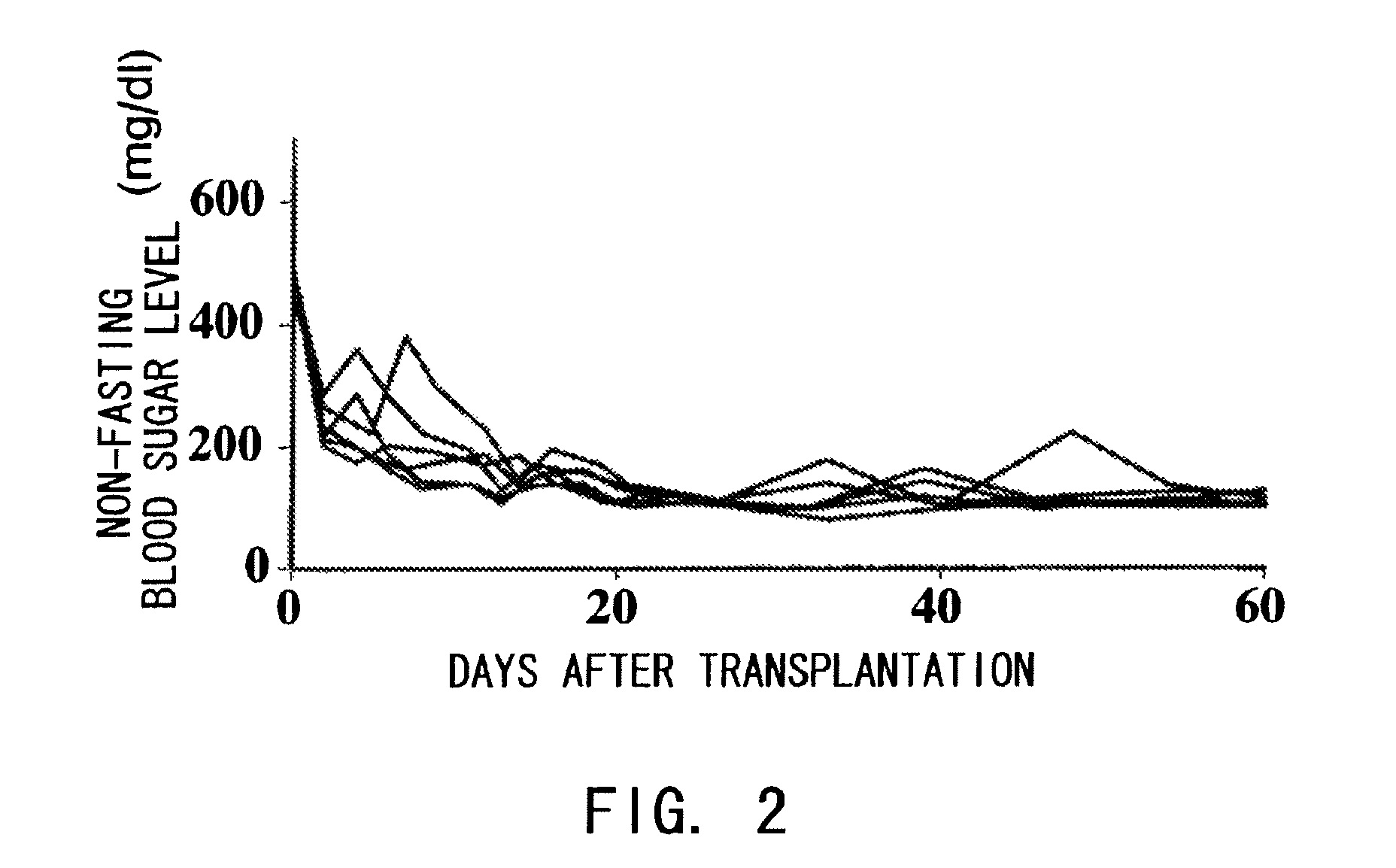
The present inventors assessed the effect of anti-IL-6 receptor antibodies in suppressing chronic rejection reaction. They assessed the effect of anti-mouse IL-6 receptor antibody (MR16-1) administration in suppressing the chronic rejection reaction using a mouse model for post-heart-transplantation chronic rejection. The result of histopathological analysis of transplanted hearts extirpated 60 days after transplantation revealed that fibrosis of myocardium and vascular stenotic lesions, which are pathological conditions characteristic of the chronic rejection reaction, were significantly suppressed in the MR16-1-treated group as compared to the control group. Thus, MR16-1 administration was demonstrated to have the effect of suppressing chronic rejection reaction. Specifically, the present inventors discovered for the first time that the rejection reaction in the chronic phase after organ transplantation was suppressed by administering an anti-IL-6 receptor antibody.
Owner:CHUGAI PHARMA CO LTD +1
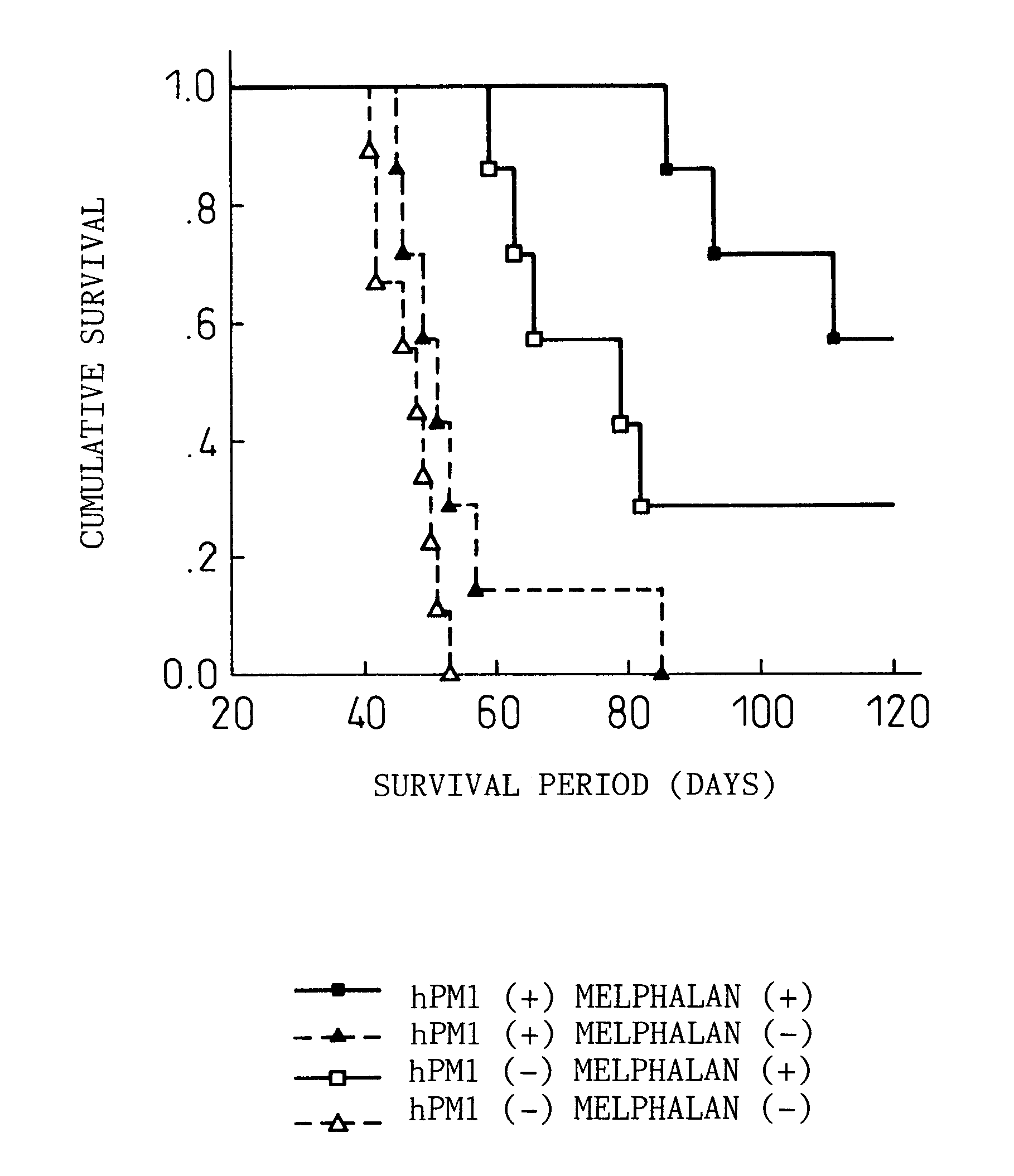
Therapeutic agent for prostate cancer
InactiveUS20090269335A1Confirm in antitumor effectTumor growth suppressedAntibody ingredientsImmunoglobulinsIL-2 receptorIn vivo
The present inventors investigated the antitumor effects of anti-IL-6 receptor antibodies against prostate cancer. The result showed that the anti-IL-6 receptor antibodies had both in vivo and in vitro antitumor effects against prostate cancer. It was also revealed that the hPM1 antitumor effect is via IL-6 receptor.
Owner:CHUGAI PHARMA CO LTD
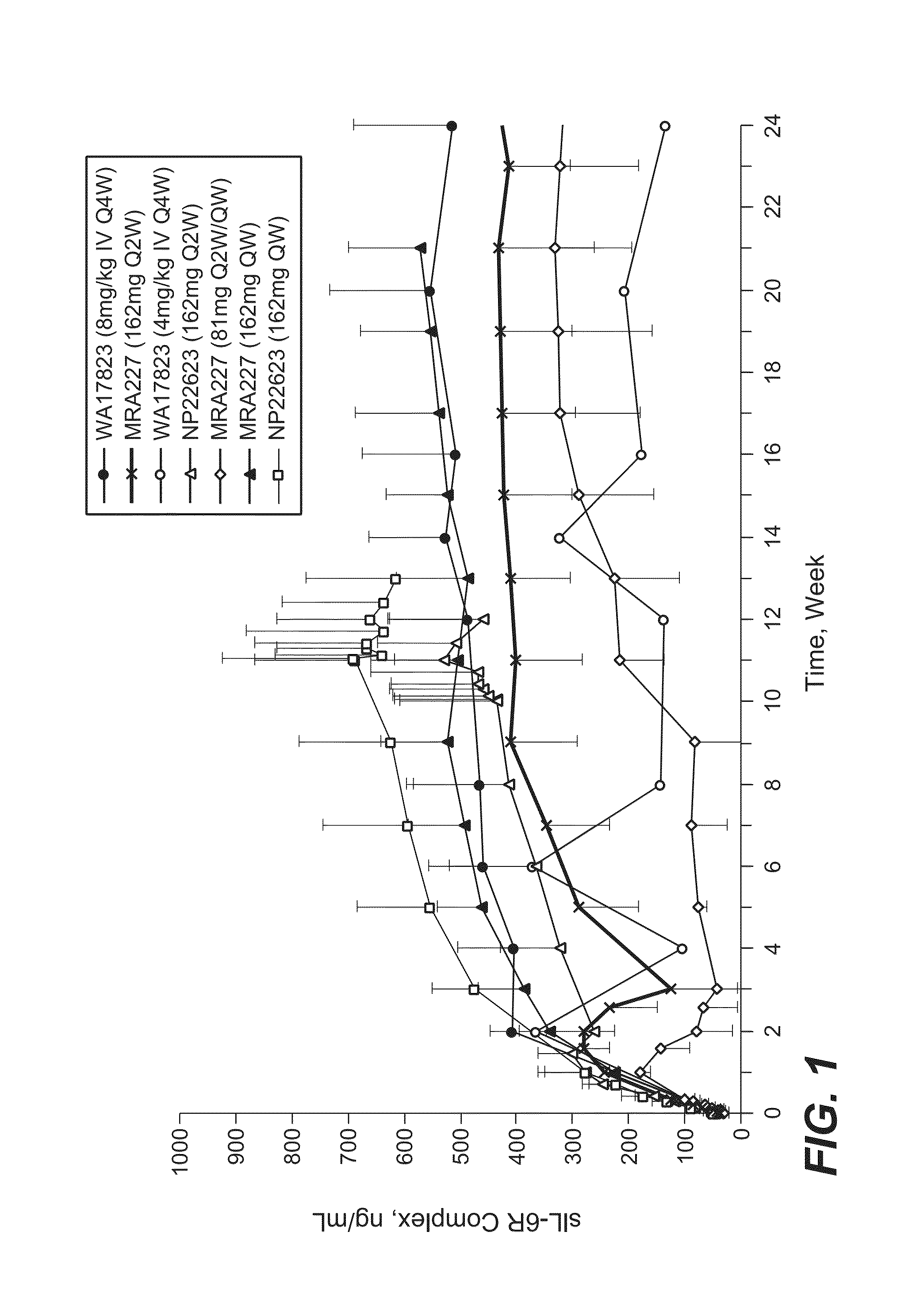
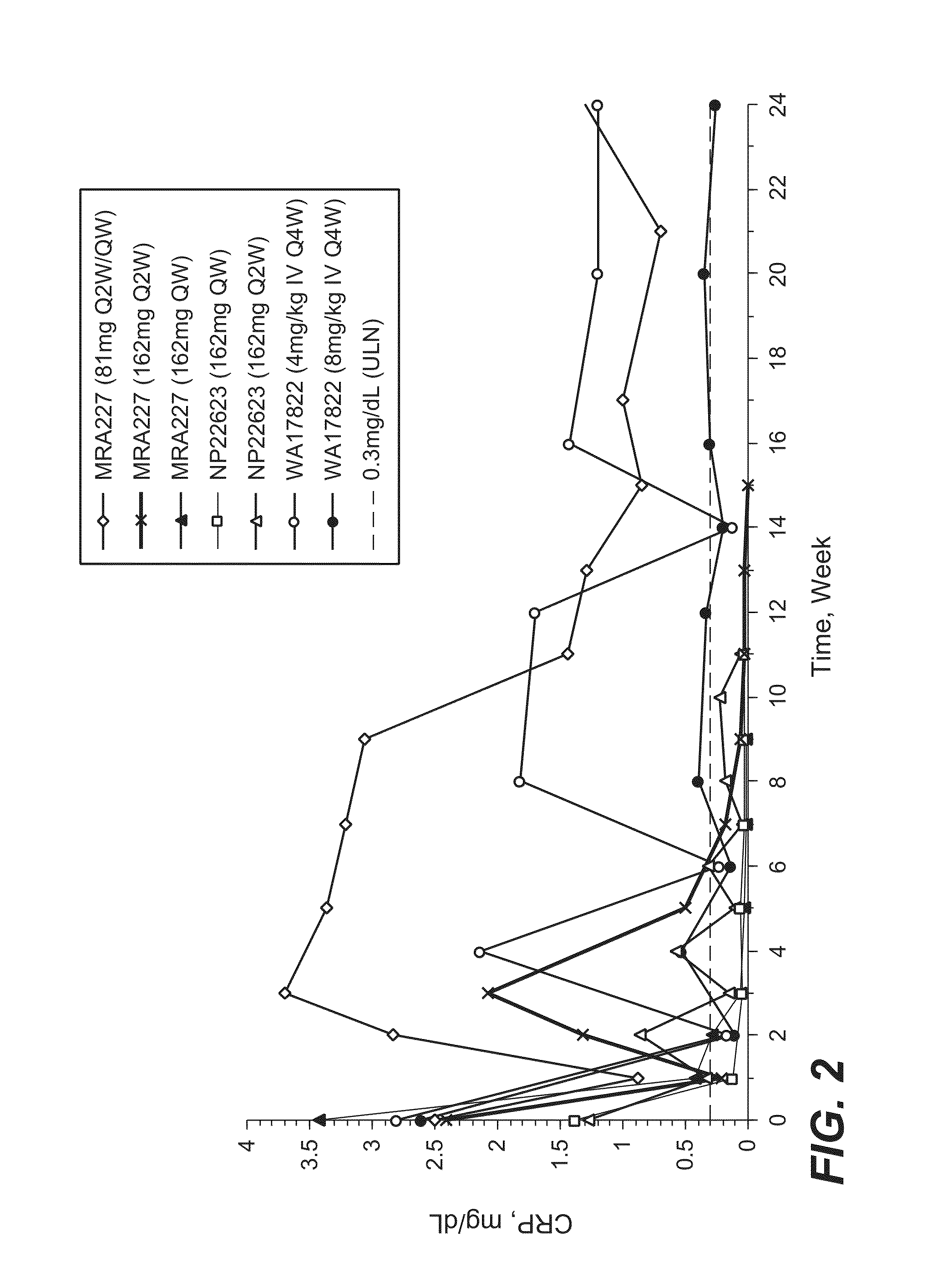
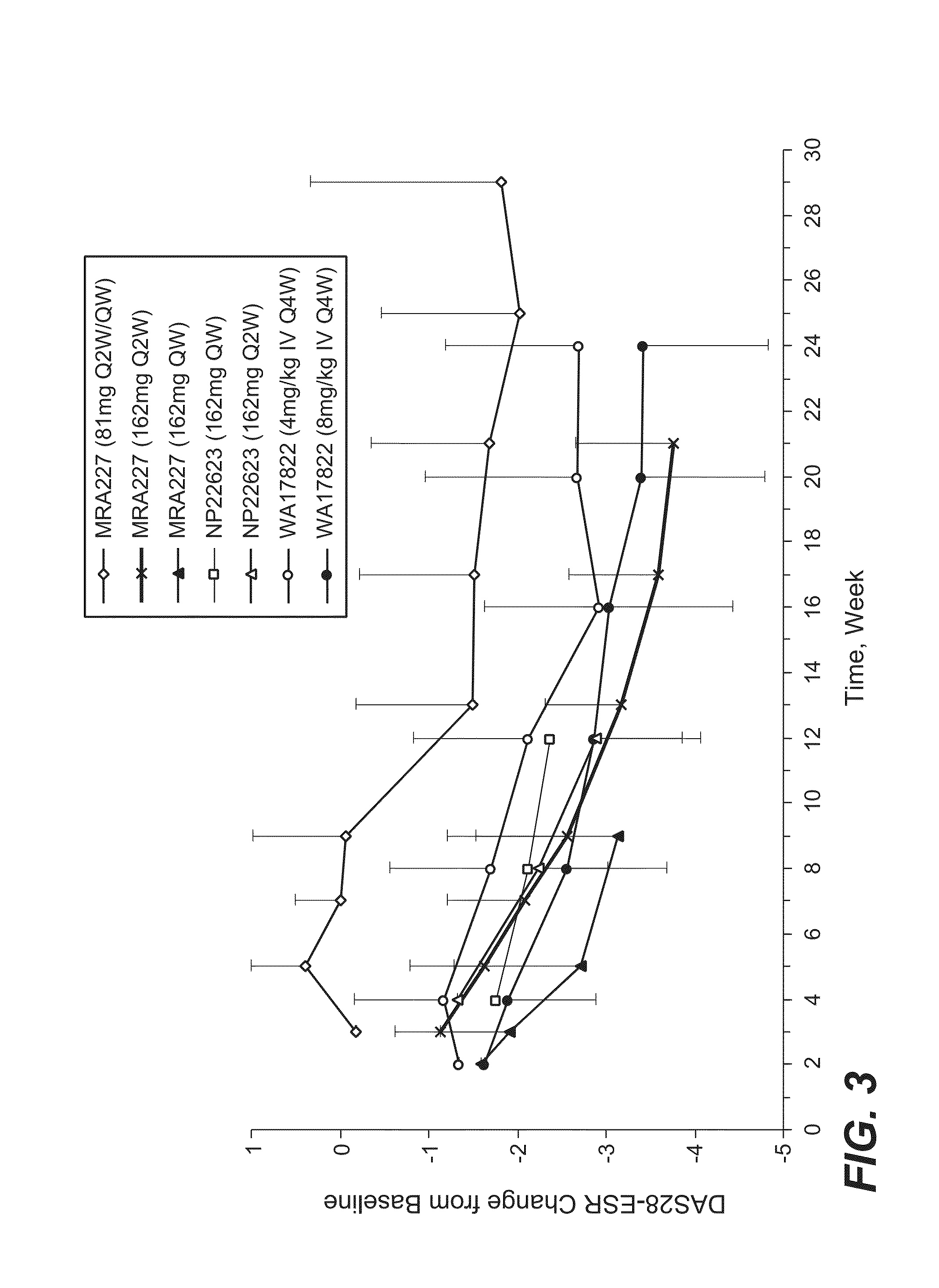
Subcutaneously administered Anti-il-6 receptor antibody
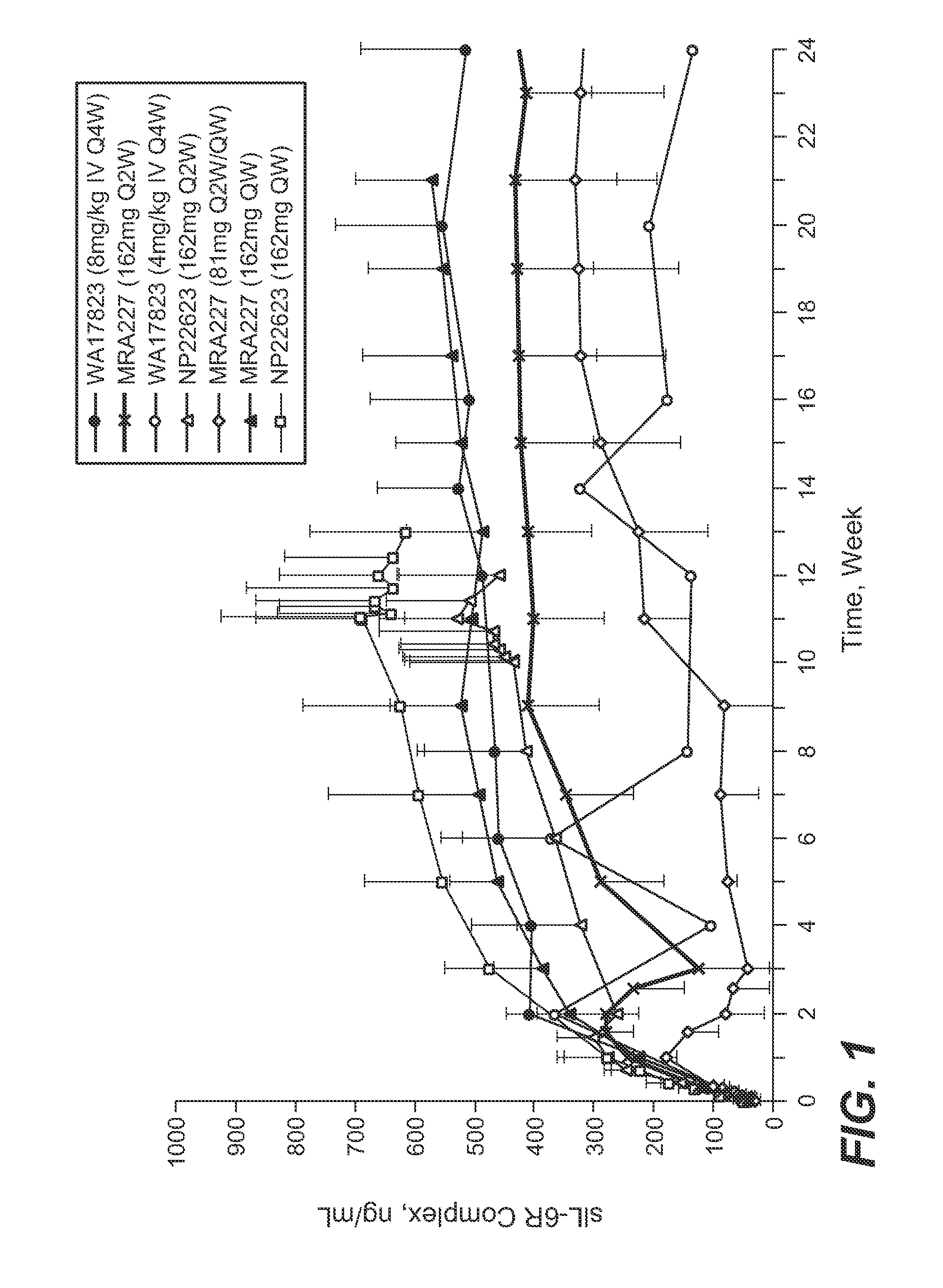
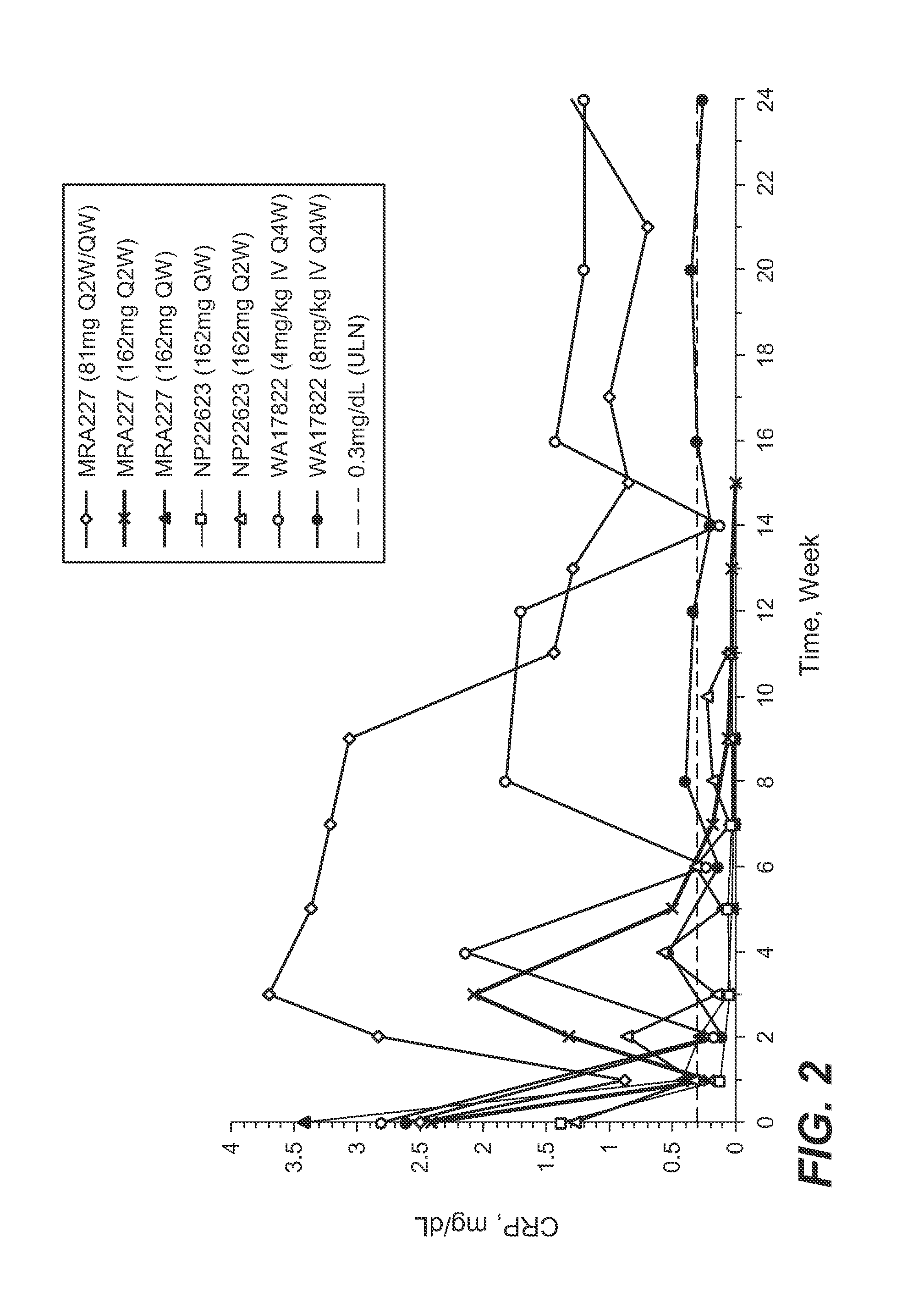
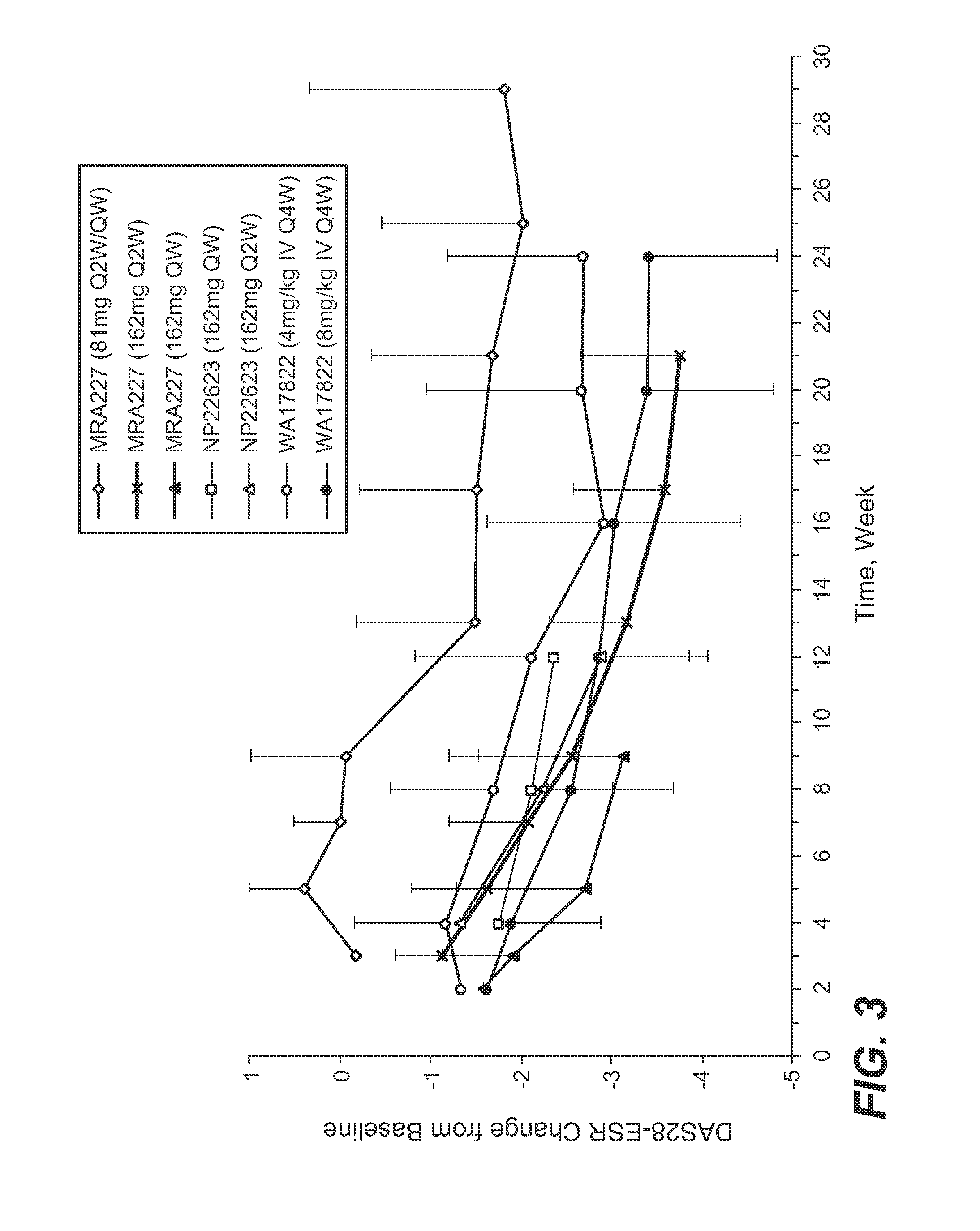
The present application discloses methods for treating an IL-6-mediated disorder such as rheumatoid arthritis (RA), juvenile idiopathic arthritis (JIA), systemic JIA (sJIA), polyarticular course JIA (pcJIA), systemic sclerosis, or giant cell arteritis (GCA), with subcutaneously administered antibody that binds interleukin-6 receptor (anti-IL-6R antibody). In particular, it relates to identification of a fixed dose of anti-IL-6R antibody, e.g. tocilizumab, which is safe and effective for subcutaneous administration in patients with IL-6-mediated disorders. In addition, formulations and devices useful for subcutaneous administration of an anti-IL-6R antibody are disclosed.
Owner:CHUGAI PHARMA CO LTD
Anti-il-6 antibodies for the treatment of anemia
ActiveUS20120128626A1Inhibition effectInhibitionBiocidePeptide/protein ingredientsCvd riskHead and neck cancer
The present invention is directed to therapeutic methods using IL-6 antagonists such as anti-IL-6 antibodies and fragments thereof having binding specificity for IL-6 to prevent or treat anemia (e.g., anemia associated with chemotherapy) including persons on a treatment regimen with a drug or chemotherapy and / or radiation for cancer (e.g., head and neck cancer) that is associated with increased risk of anemia.
Owner:ALDERBIO HLDG LLC +1
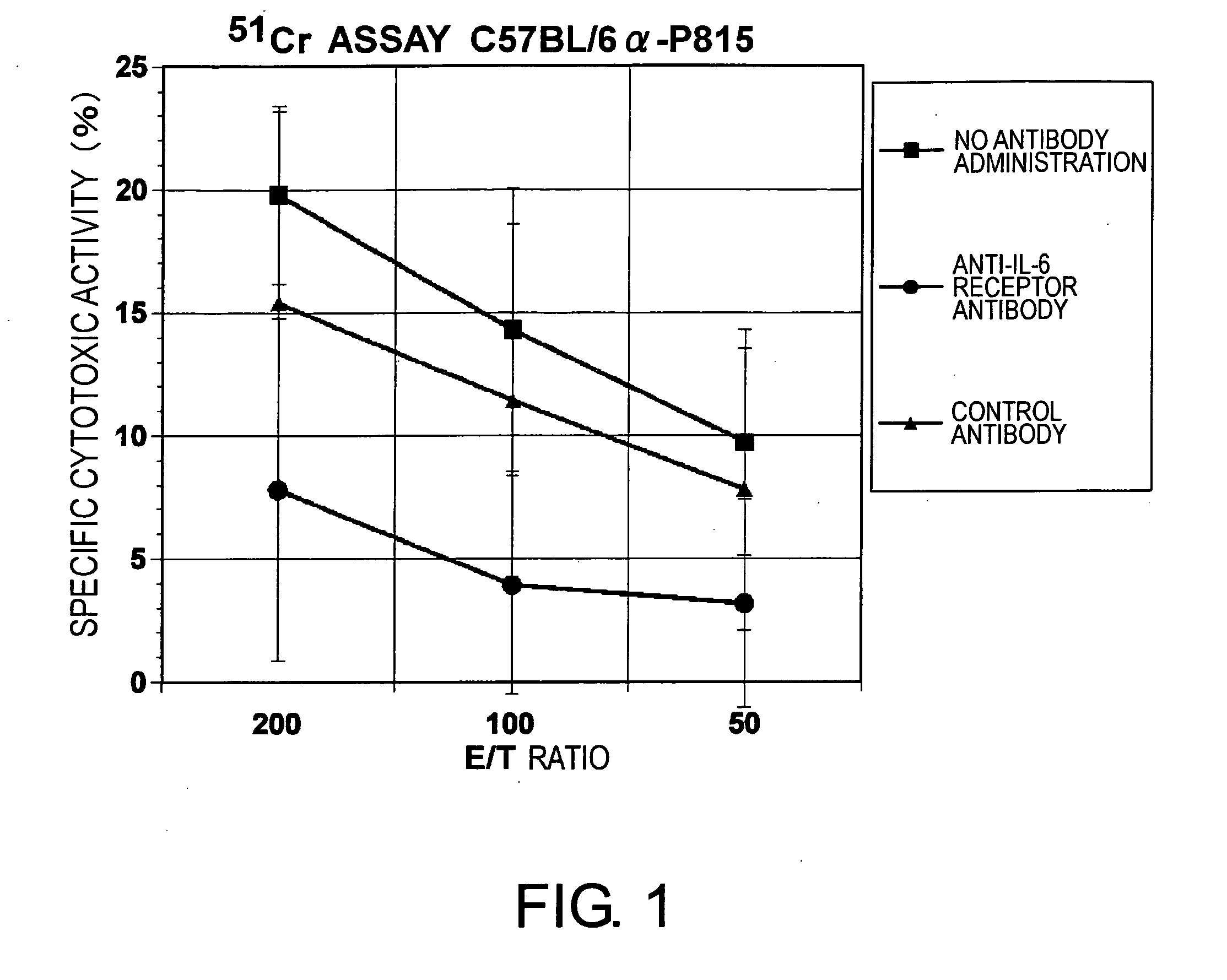
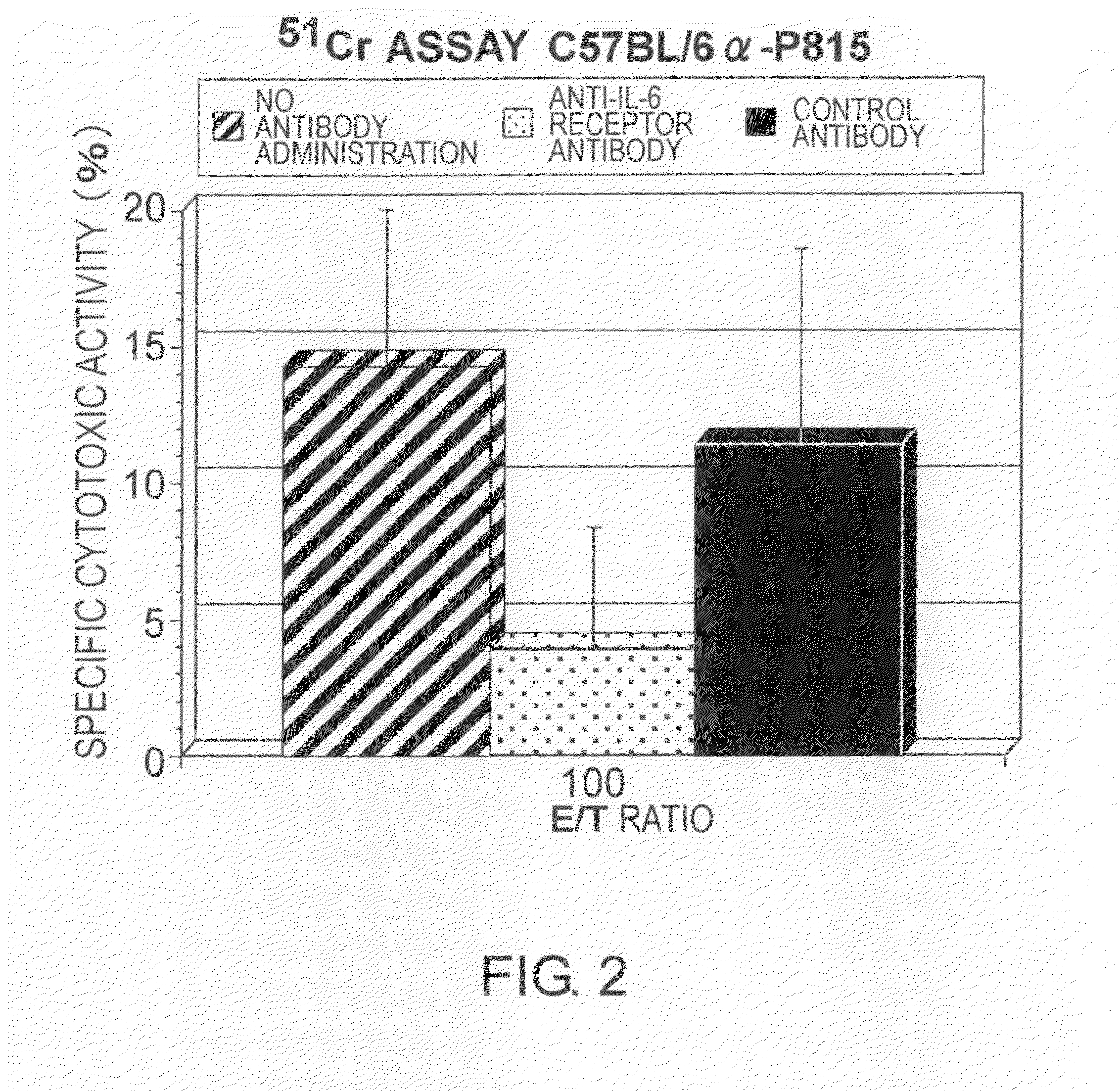
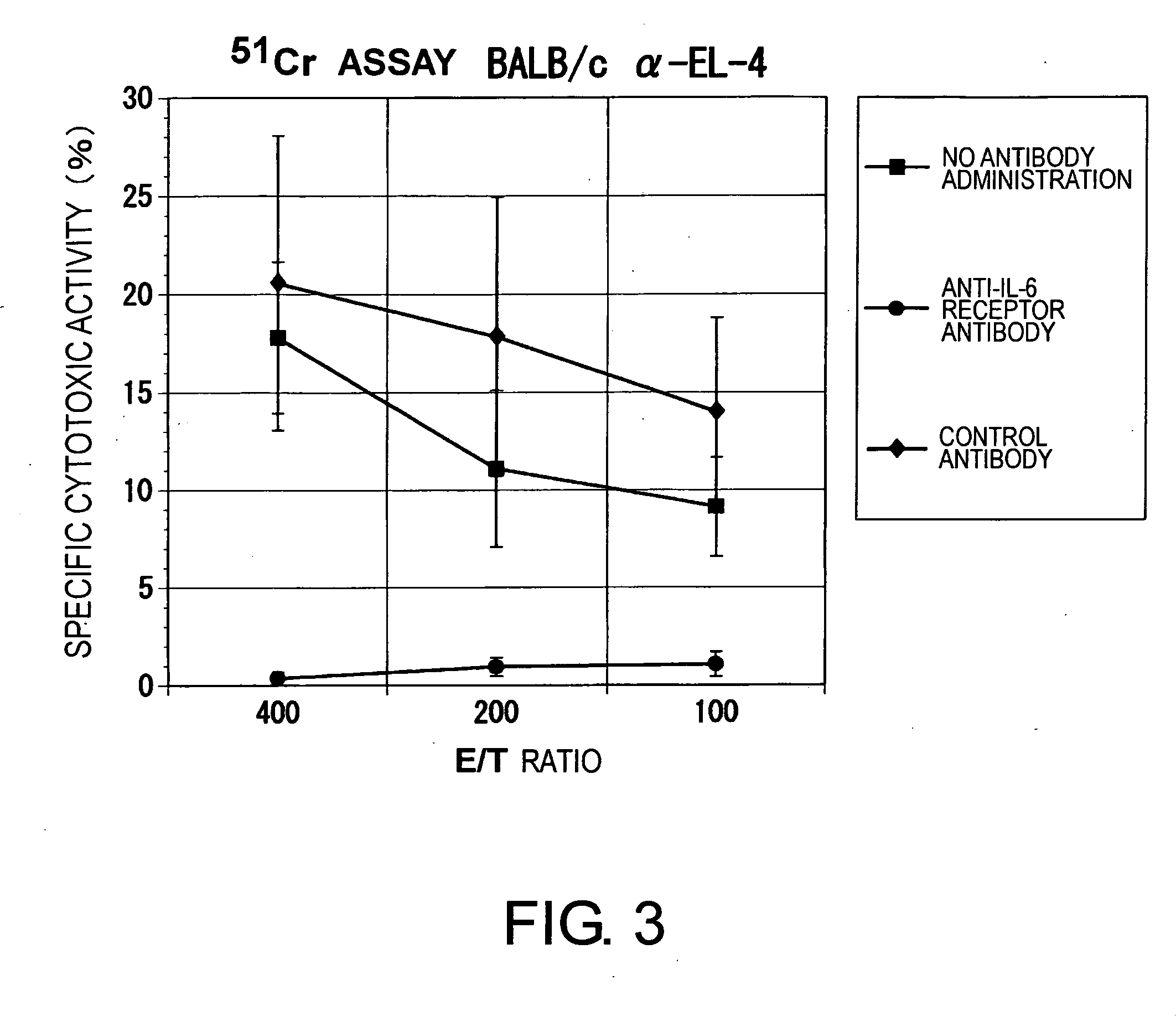
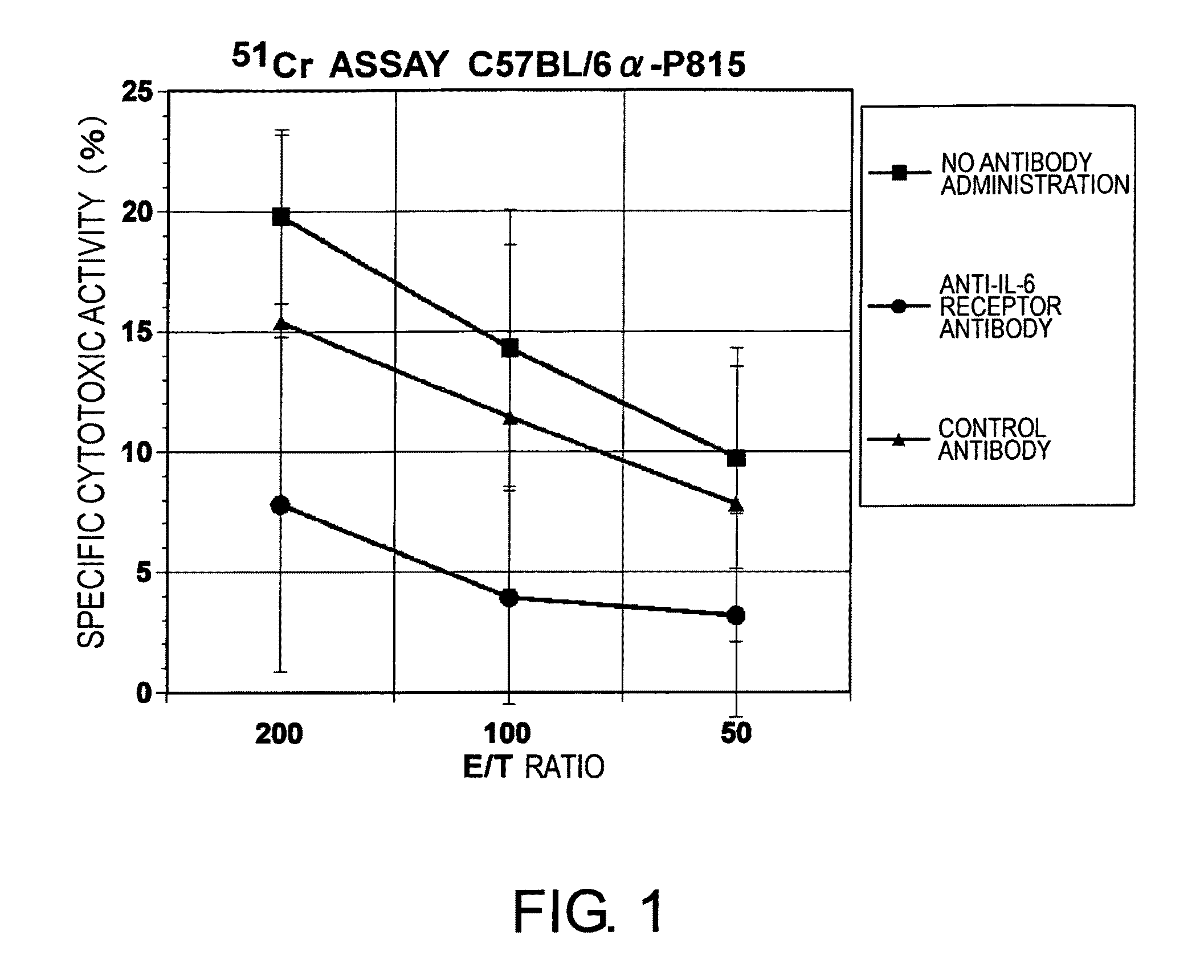
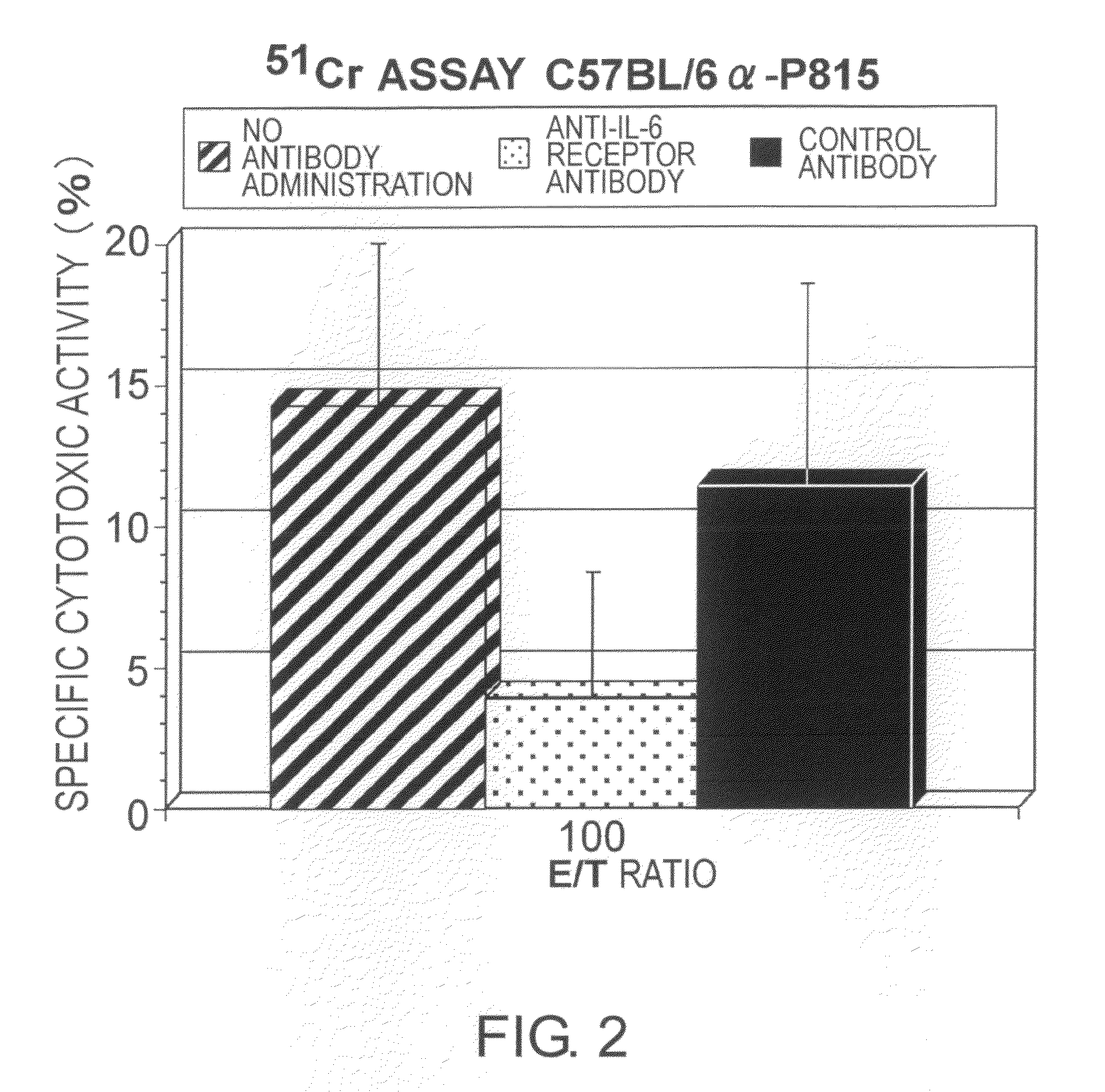
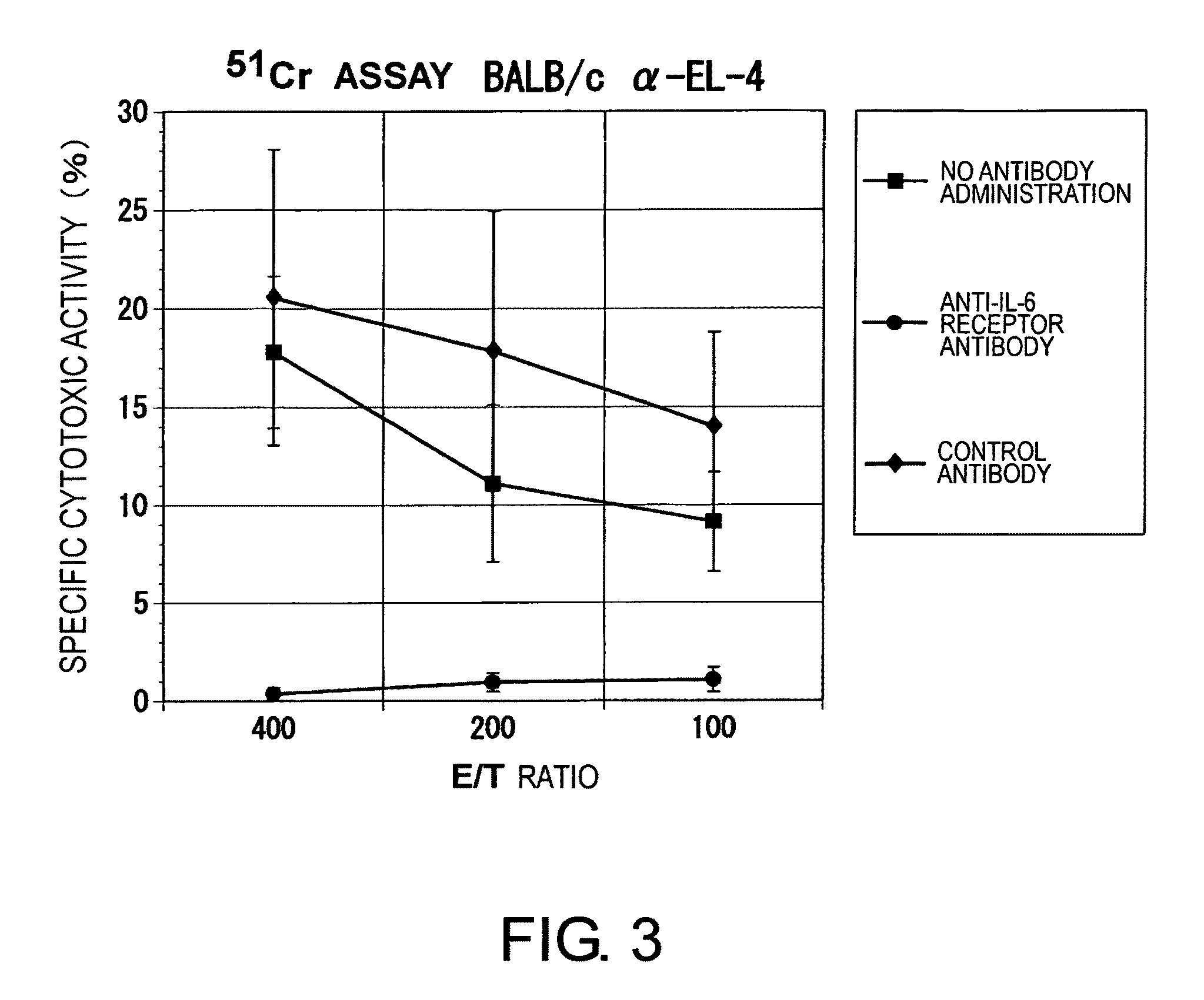
Agents for Suppressing the Induction of Cytotoxic T Cells
ActiveUS20090263384A1Suppressed acute heart transplant rejectionImprove survivalSurgical drugsAntibody ingredientsHistopathology findingTransplanted heart
The effect of anti-IL-6 receptor antibodies in suppressing cytotoxic T cell induction was examined. The results showed that CTL activity against alloantigens was statistically significantly reduced in mice treated with anti-IL-6 receptor antibodies as compared to mice not treated with antibodies and mice treated with a control antibody. The anti-IL-6 receptor antibody was also administered to recipients of a mouse model for allogenic heart transplantation. As a result, histopathological findings showed that inflammatory cell infiltration into transplanted hearts was suppressed and the survival period of transplanted hearts was significantly prolonged. Thus, the present inventors for the first time discovered that administration of anti-IL-6 receptor antibodies could suppress cytotoxic T cell induction and thereby suppress acute rejection after transplantation.
Owner:CHUGAI PHARMA CO LTD +2
Anti-IL-6 antibodies, compositions, methods and uses
The present invention relates to at least one novel chimeric, humanized or CDR-grafted anti-IL-6 antibodies derived from the murine CLB-8 antibody, including isolated nucleic acids that encode at least one such anti-IL-6 antibody, vectors, host cells, transgenic animals or plants, and methods of making and using thereof, including therapeutic compositions, methods and devices.
Owner:CENTOCOR
Agents for treating cardiopathy
ActiveUS20090220500A1Improve the situationSuppressing left ventricular remodelingAntibody ingredientsImmunoglobulinsCardiac echoLeft ventricular size
The present inventors investigated the effects of anti-IL-6 receptor antibodies on improving the condition of infarcted areas in myocardial infarction, and on suppressing left ventricular remodeling after myocardial infarction. As a result, the administration of anti-IL-6 receptor antibodies significantly suppressed the increase of MPO activity in the infarcted area and suppressed myocardial MCP-1 expression in both the infarcted area and the non-infarcted area. Furthermore, echocardiography and histological examinations revealed that cardiac hypertrophy is also suppressed.
Owner:CHUGAI PHARMA CO LTD
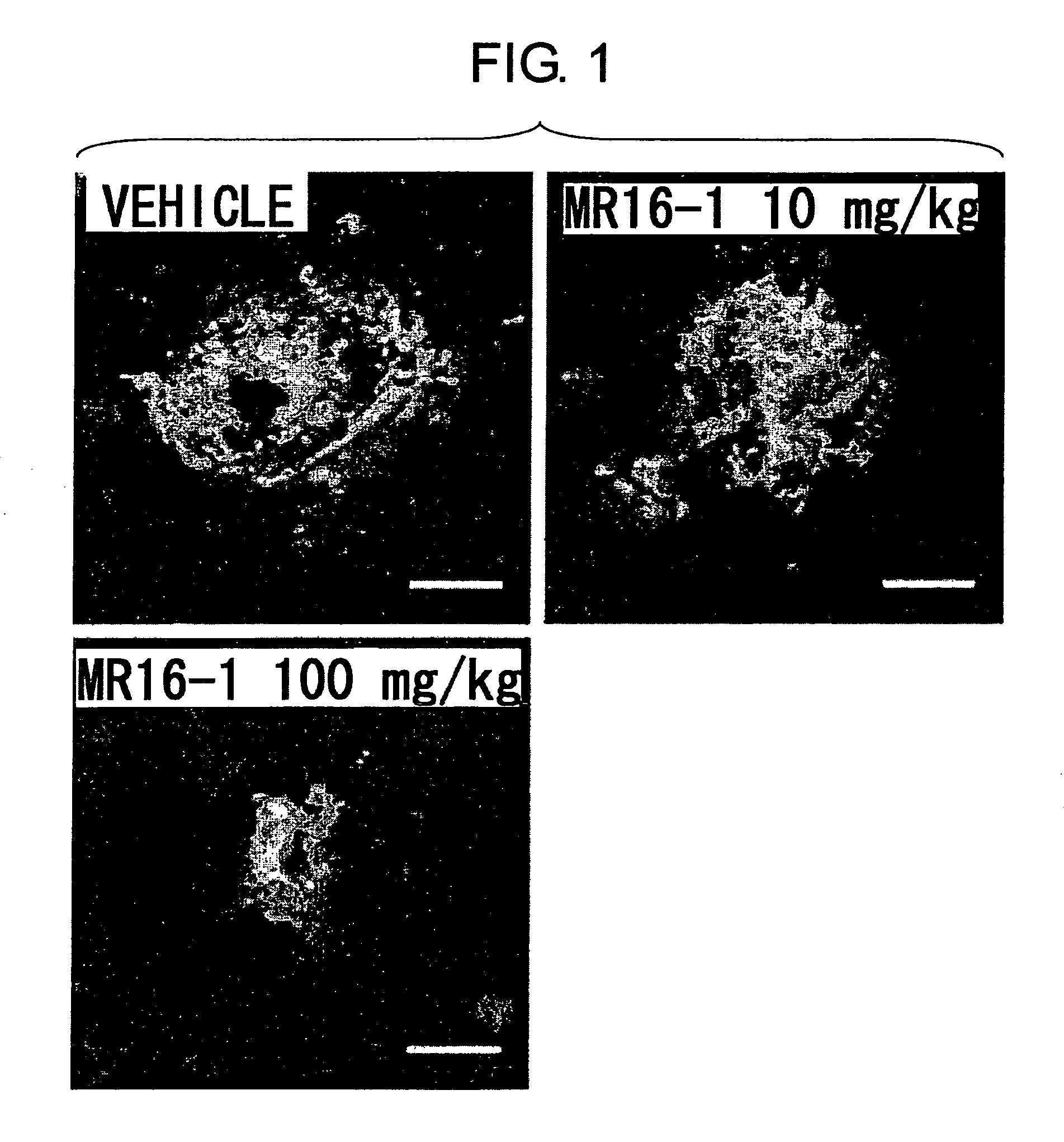
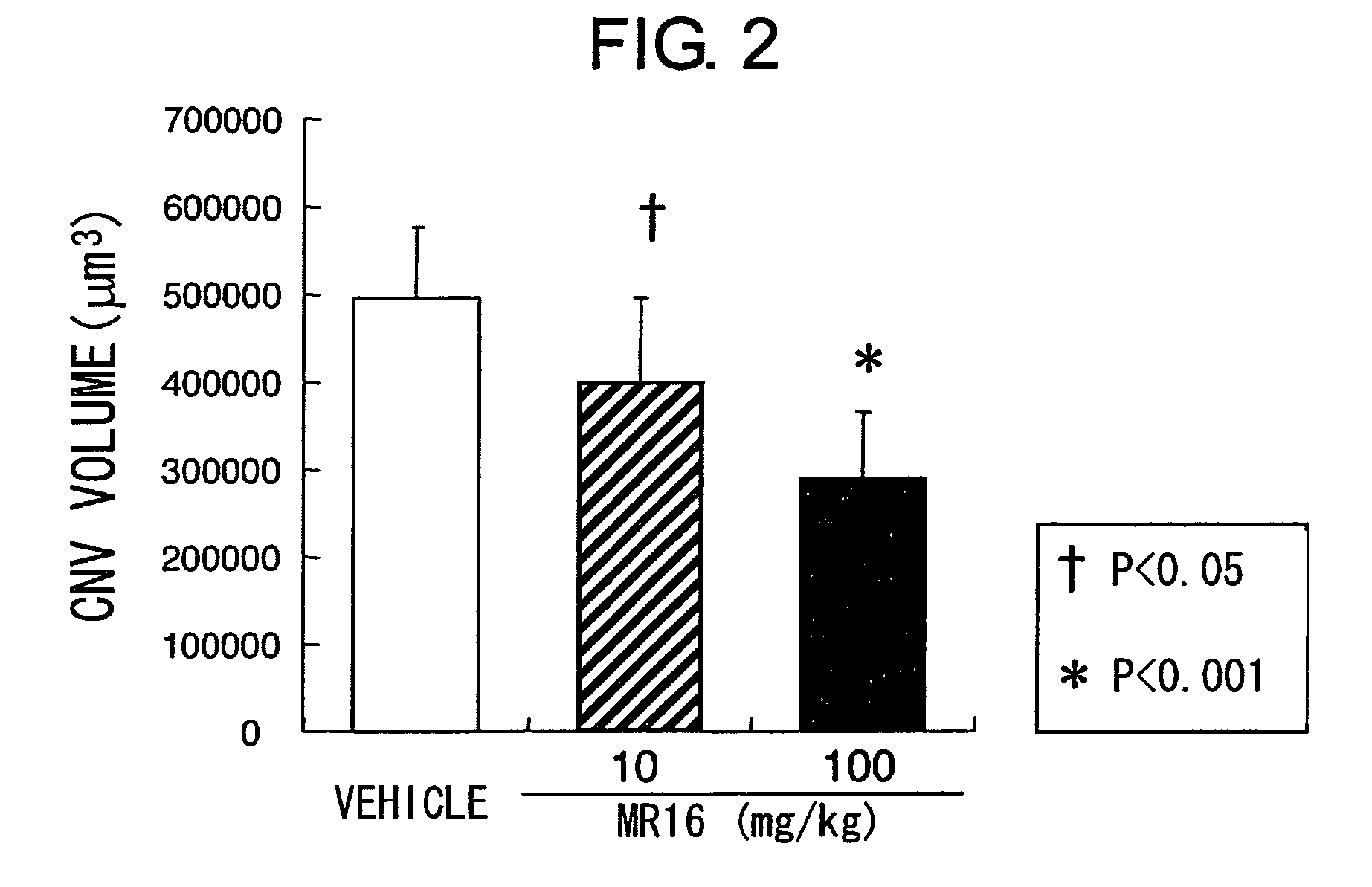
Therapeutic agents for diseases involving choroidal neovascularization
ActiveUS20100034811A1Advancement of CNV could be suppressedSenses disorderAntibody ingredientsDiseaseNeovascularization
The present inventors focused on the fact that inflammation at the subretinal macular area enhances choroidal neovascularization, and developed pharmaceutical agents that suppress initiation or advancement of neovascularization by angiogenic factors such as VEGF. More specifically, the present inventors revealed that administering anti-IL-6 receptor monoclonal antibodies to mice treated with laser photocoagulation inhibits the development of choroidal neovascularization.
Owner:CHUGAI PHARMA CO LTD +1
Cancer Metastasis Inhibitor
InactiveUS20120183539A1Promoted angiogenesisPromoted tumor growthImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsHematopoietic cellLymphatic Spread
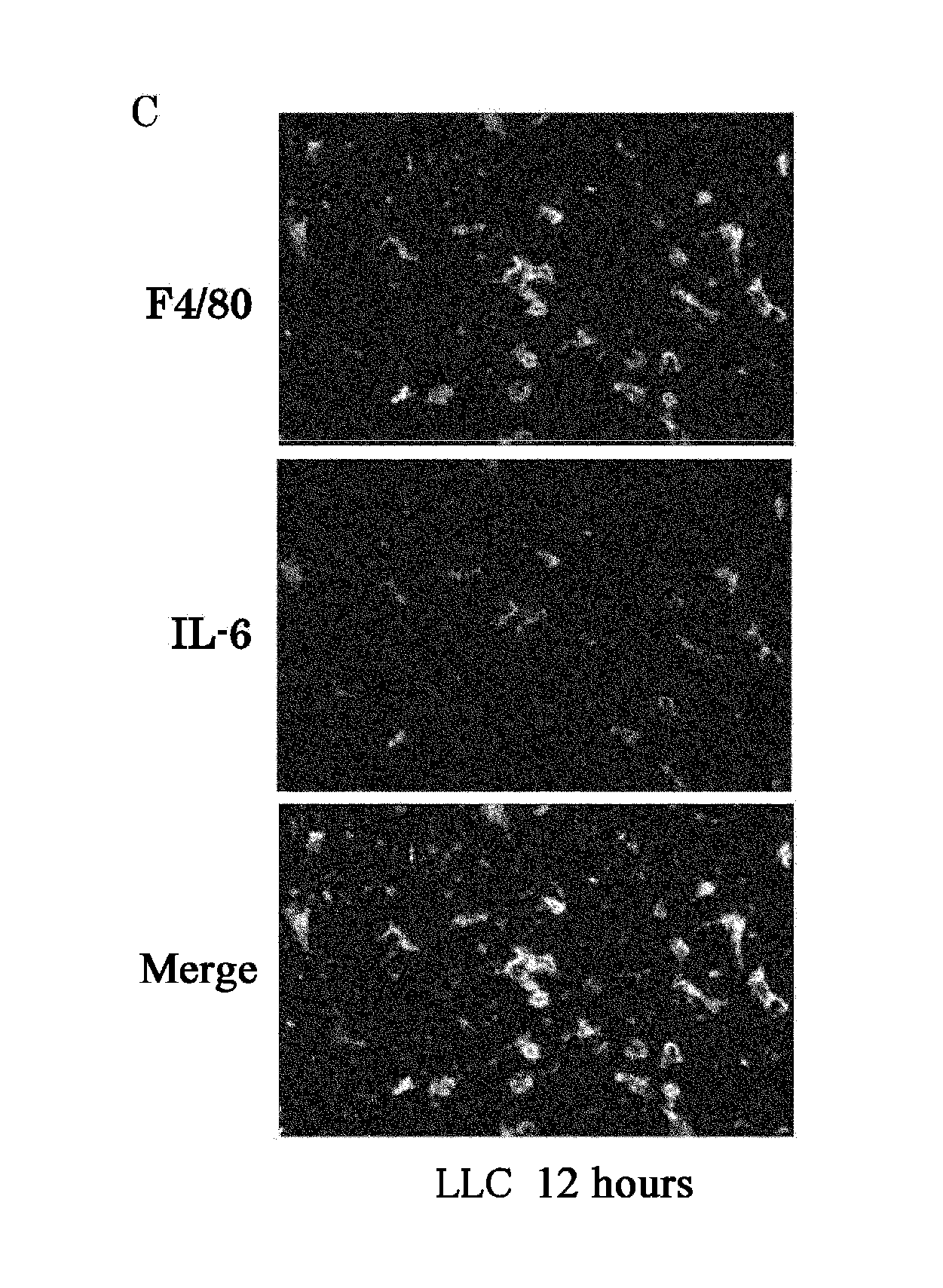
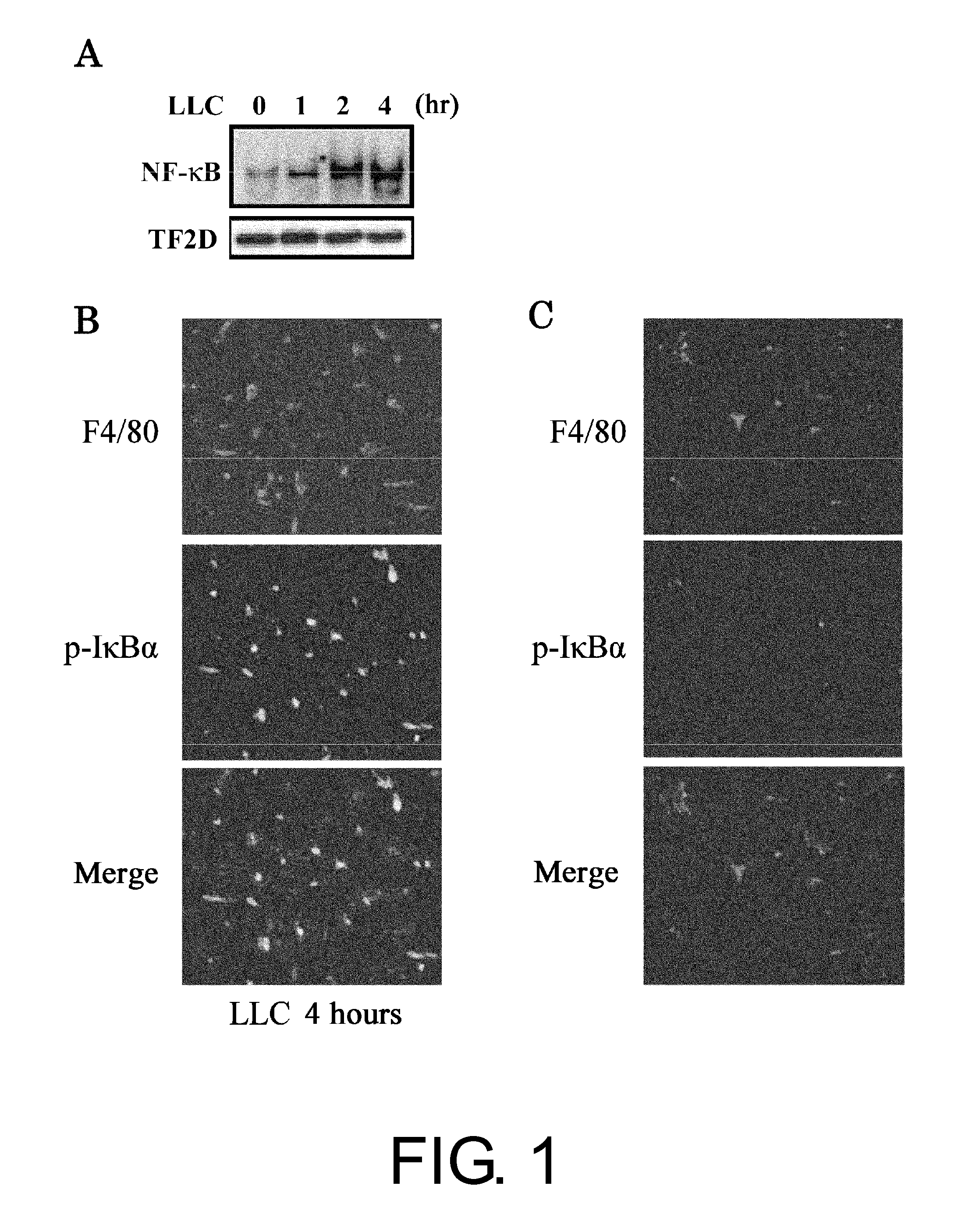
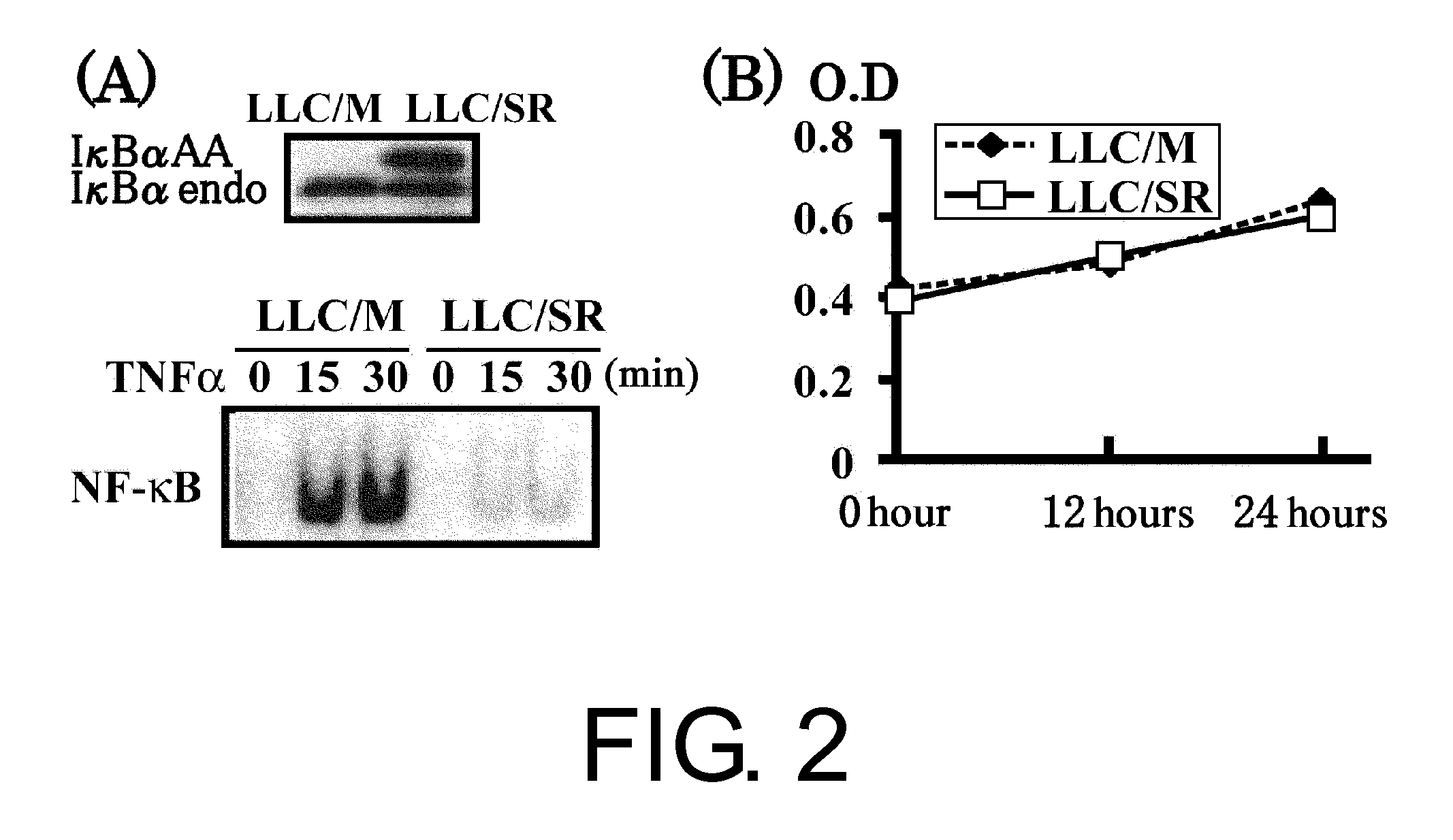
The present inventors used a model of intrasplenically induced liver metastasis to determine whether or not NF-κB activation in the liver is involved in the onset of metastatic tumors. When IKKβ was deleted from both liver cells and hematopoietically-derived cells, the onset of tumors was reduced remarkably. Tumor cells activated neighboring bone marrow cells (Kupffer cells) and produced mitogens such as interleukin (IL)-6, and this promoted angiogenesis and growth of tumors. The mitogen production depended on NF-κB in hematopoietically-derived Kupffer cells. Furthermore, treatment with an anti-IL-6 receptor antibody decreased the degree of metastatic tumor development. That is, the present inventors showed that tumor metastasis depends on inflammation, and proinflammatory intervention that targets Kupffer cells is useful for chemical prevention of metastatic tumors. Furthermore, it was shown that inhibition of the IKKβ / NF-κB signal transduction pathway, in particular IL-6 inhibition, can be utilized for anti-metastasis agents.
Owner:MAEDA CORPORATION
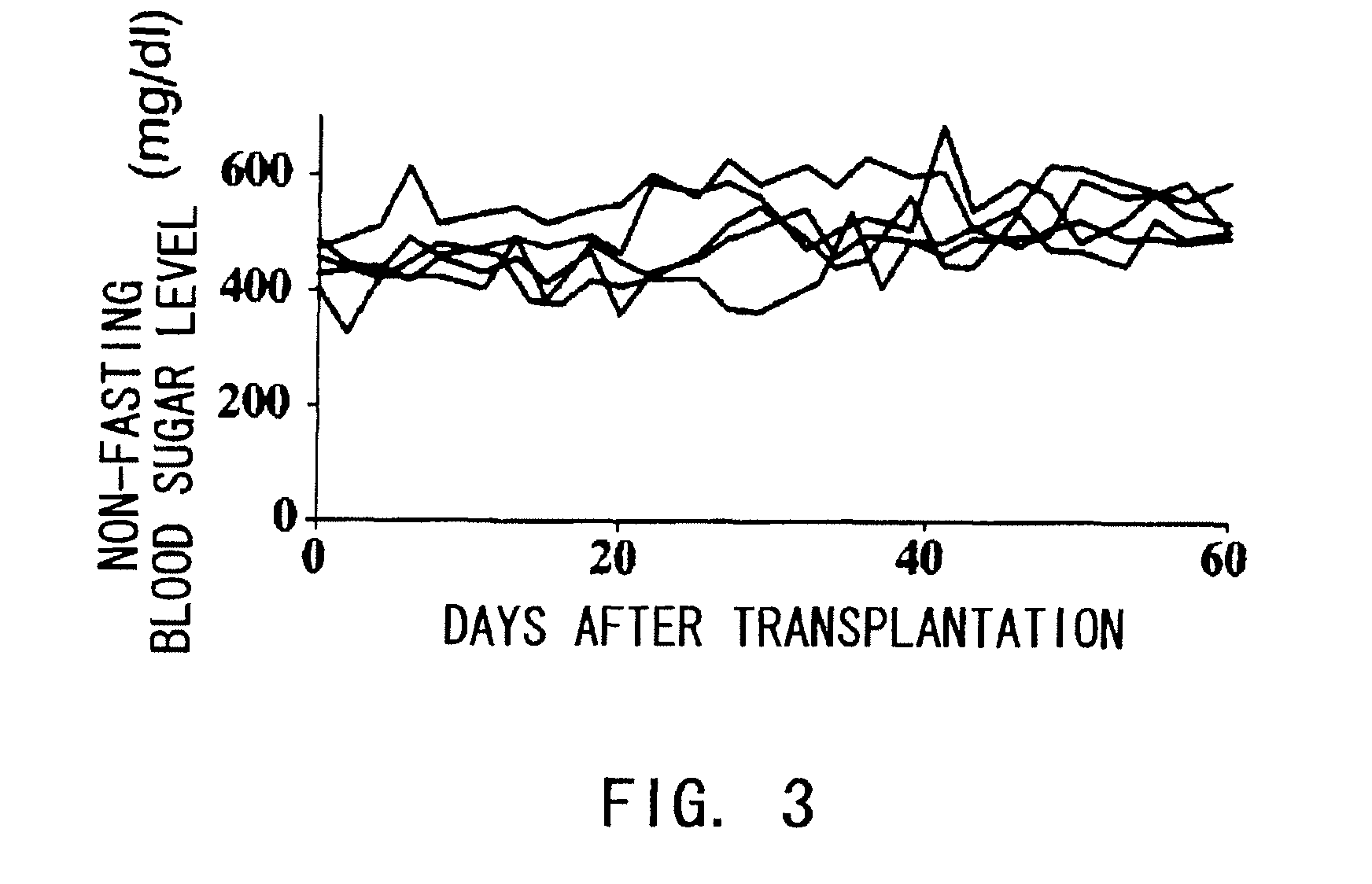
Agents for Suppressing Damage to Transplanted Islets After Islet Transplantation
ActiveUS20090220499A1Improve islet viabilityDamage suppressionAntipyreticMetabolism disorderAnti-IL-6Cytokine
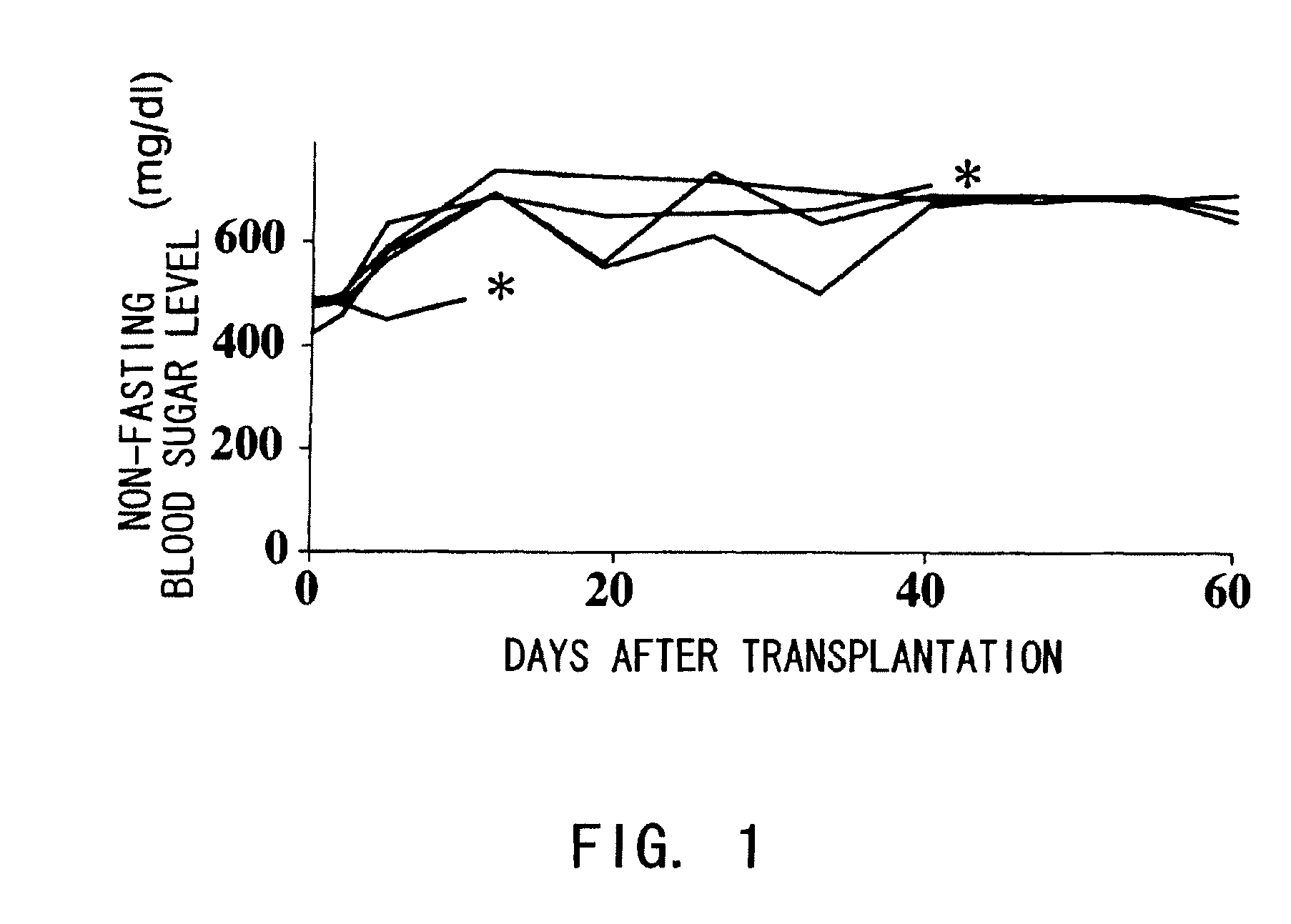
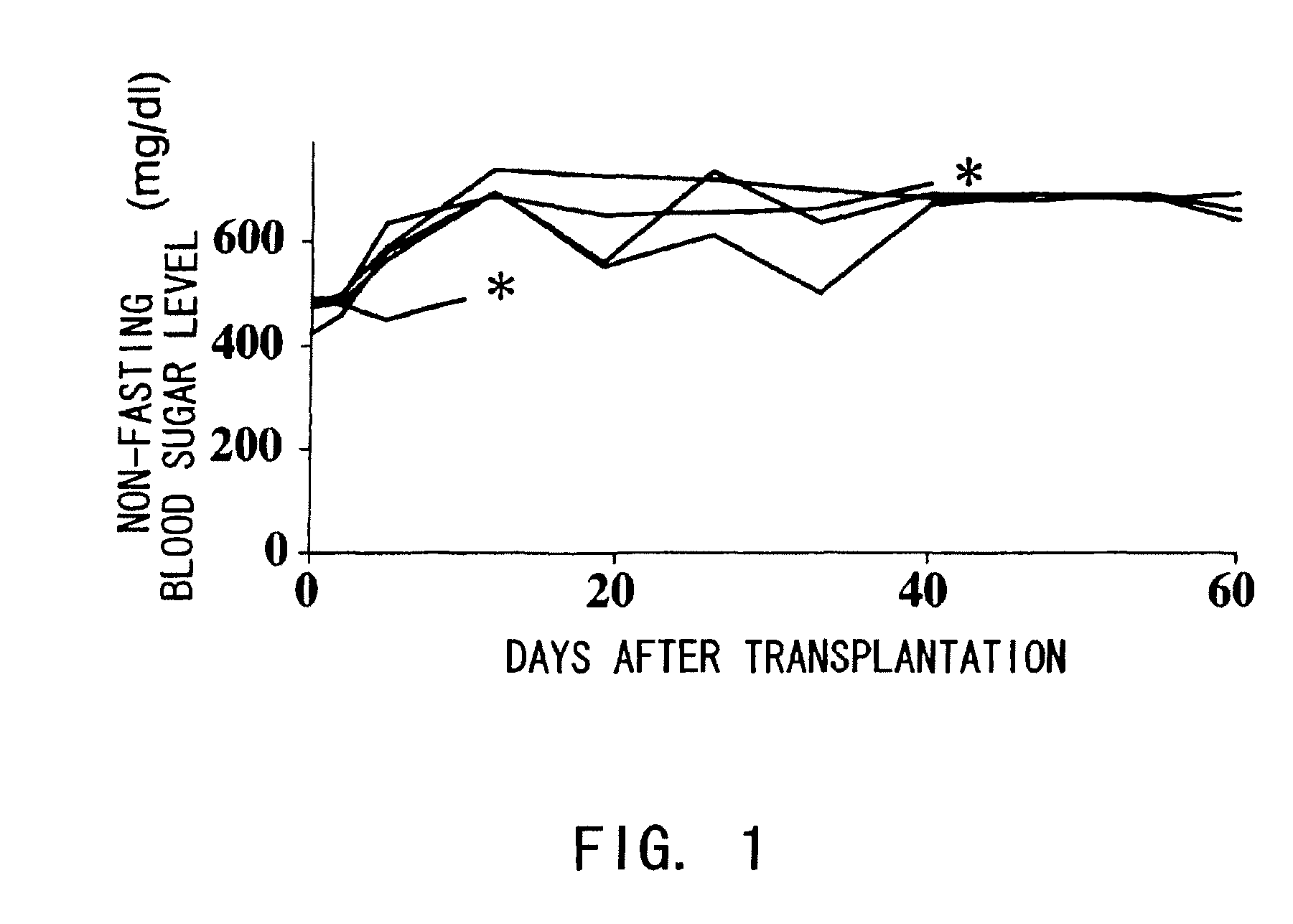
The present inventors investigated anti-IL-6 receptor antibodies for their effect in suppressing damage to transplanted islets after islet transplantation. As a result, they found that anti-IL-6 receptor antibodies reduced damage to transplanted islets, improved islet survival, and corrected hyperglycemia in recipients. Further, they revealed that administration of the anti-IL-6 receptor antibodies of the present invention suppressed the production of inflammatory cytokines by infiltrating cells after transplantation. Specifically, the present inventors discovered for the first time that damage to transplanted islets after islet transplantation can be suppressed by using anti-IL-6 receptor antibodies according to the present invention.
Owner:CHUGAI PHARMA CO LTD
Anti-il-6 antibodies for the treatment of oral mucositis
ActiveUS20120189629A1InhibitionEasy to keepOrganic active ingredientsPeptide/protein ingredientsIncreased riskCvd risk
Owner:VITAERIS INC +1
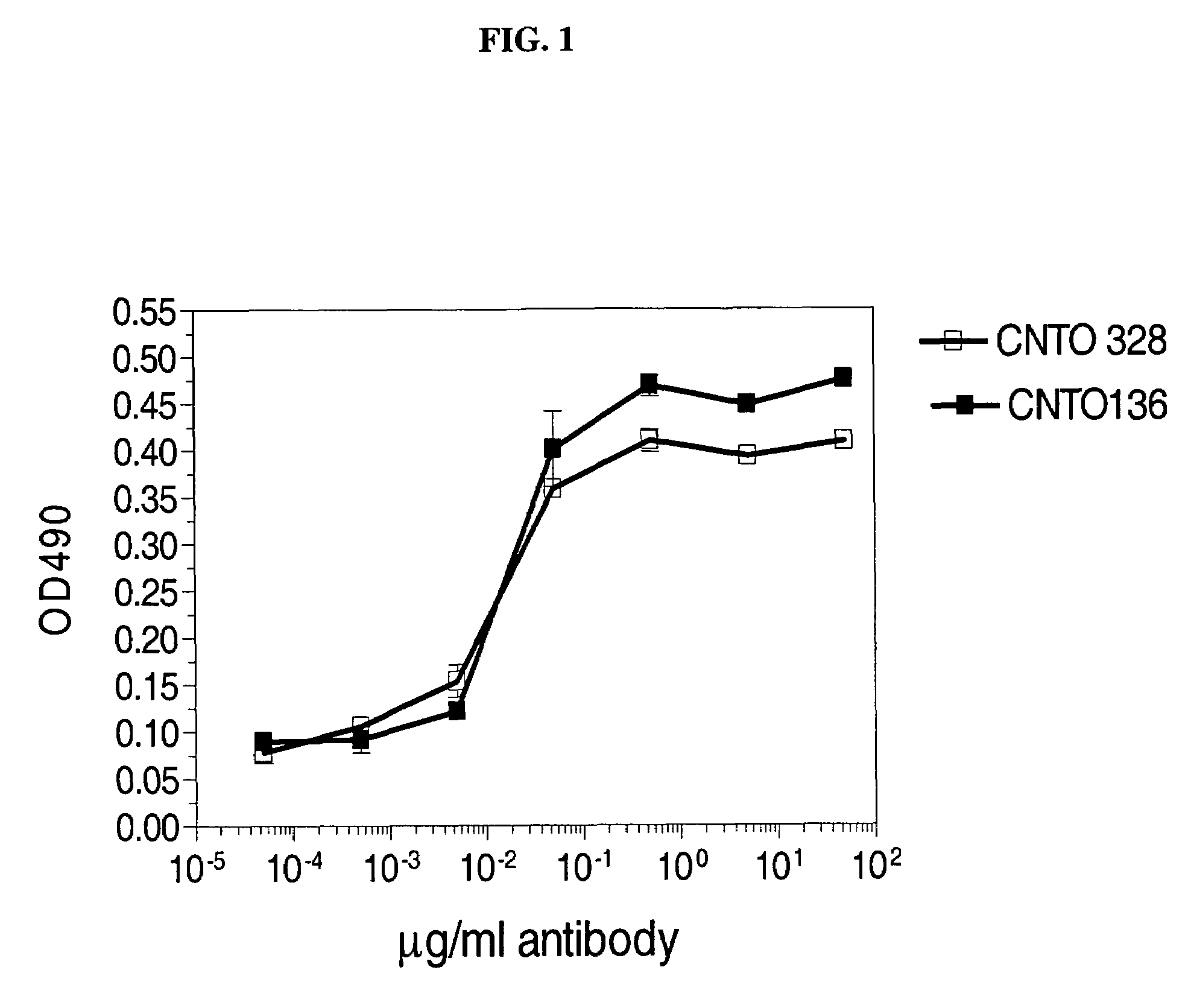
Antibody molecules that bind to il-6 receptor
InactiveUS20120253016A1Good curative effectImprove pharmacokineticsFungiSenses disorderTherapeutic effectIL-2 receptor
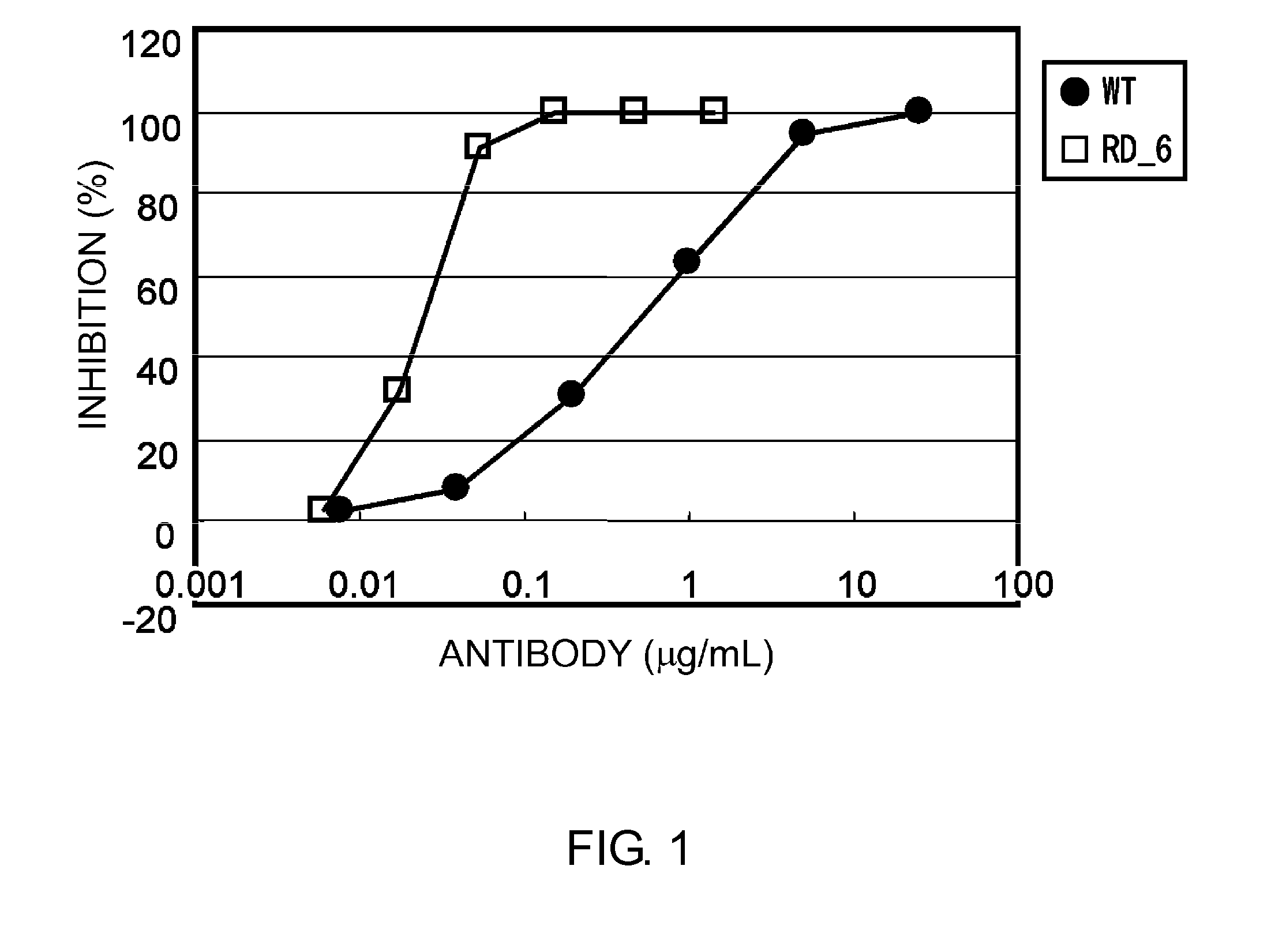
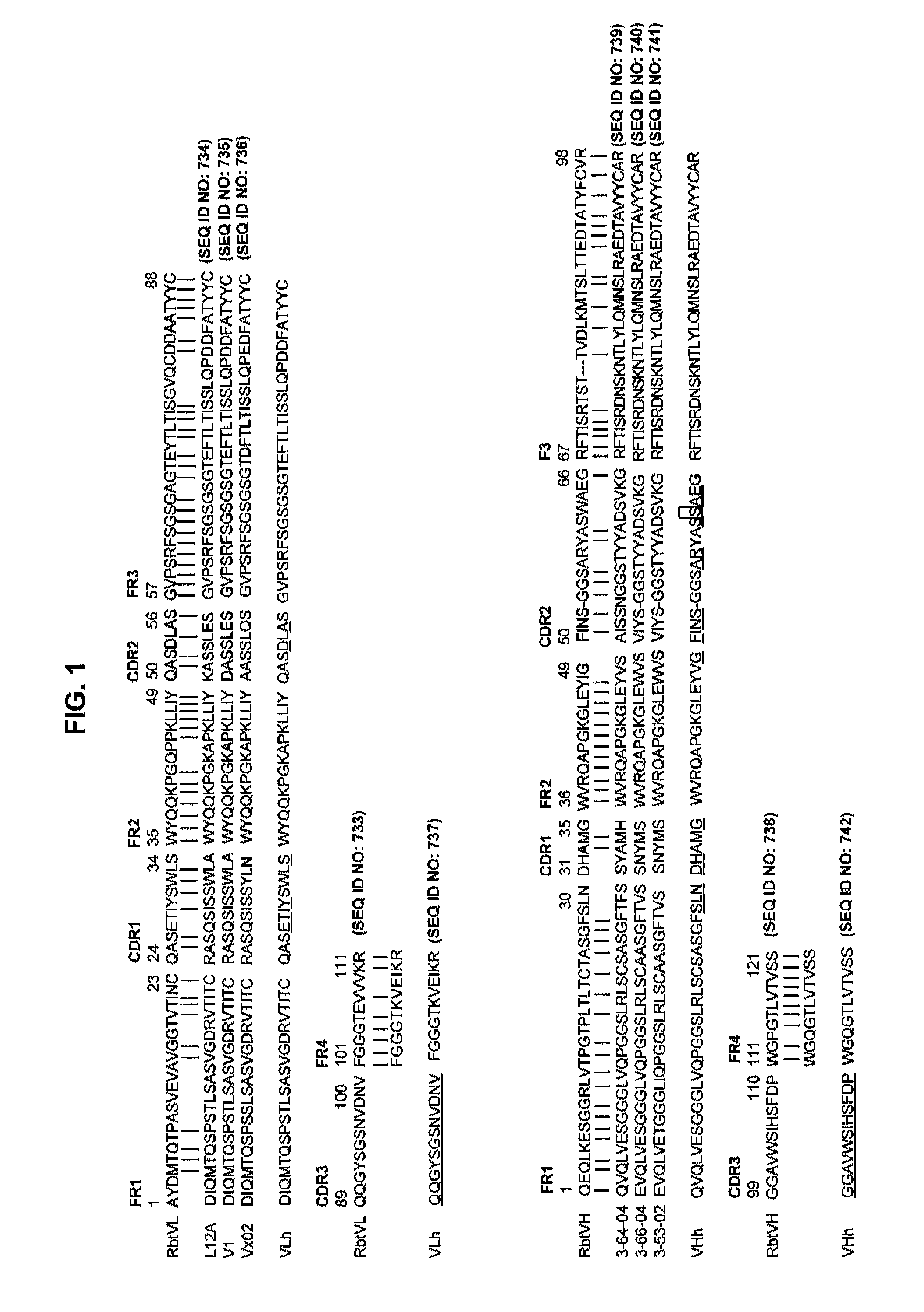
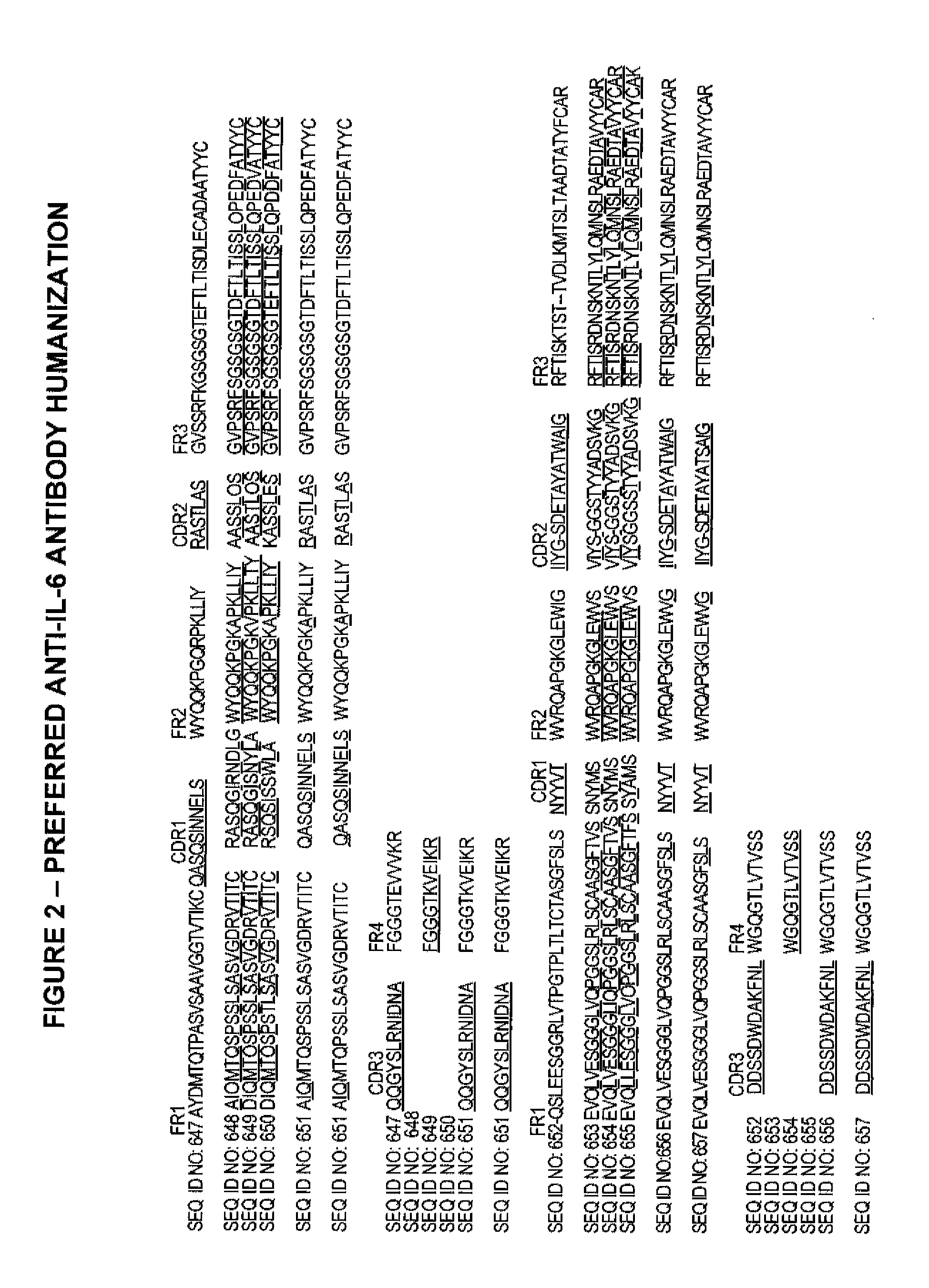
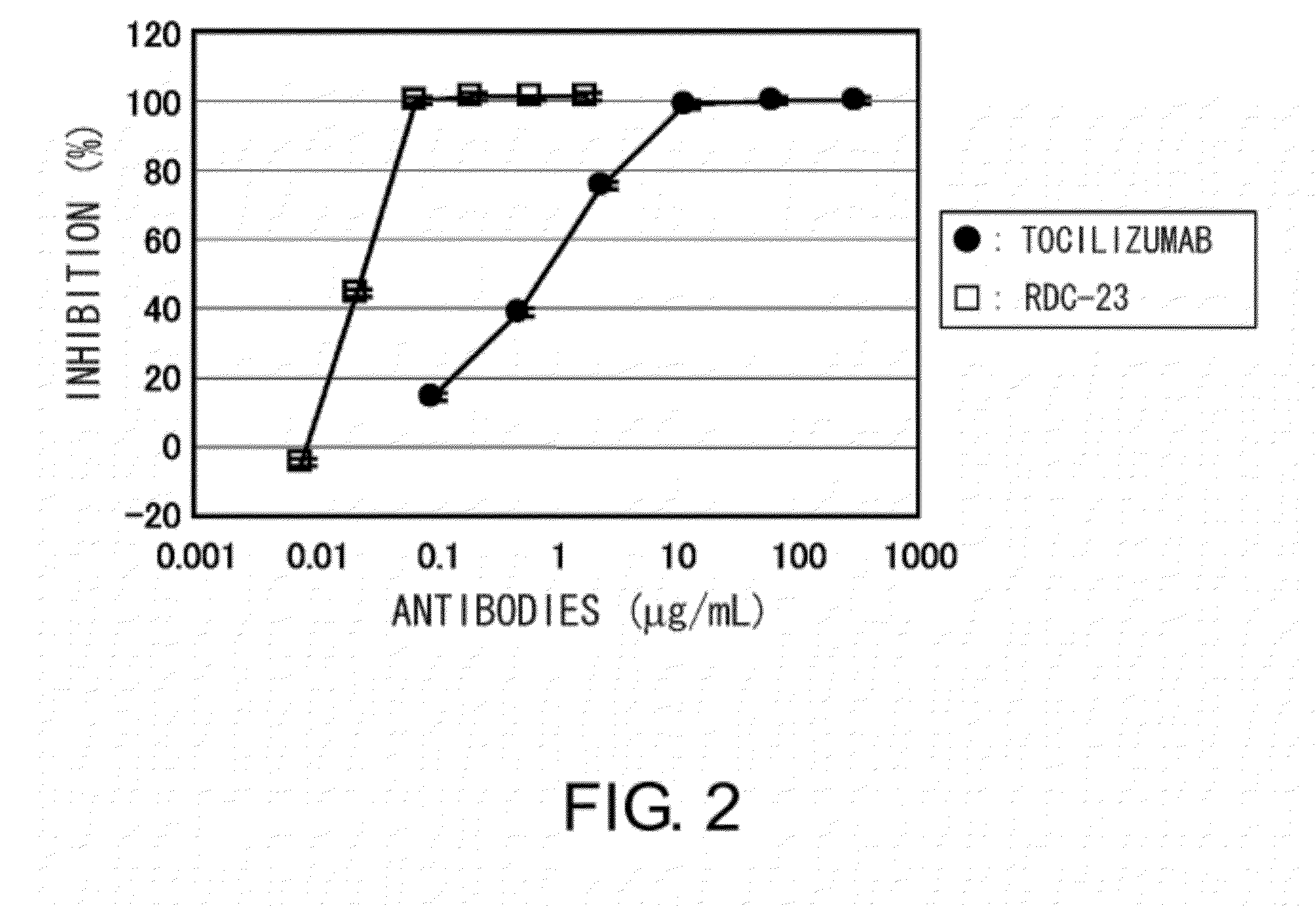
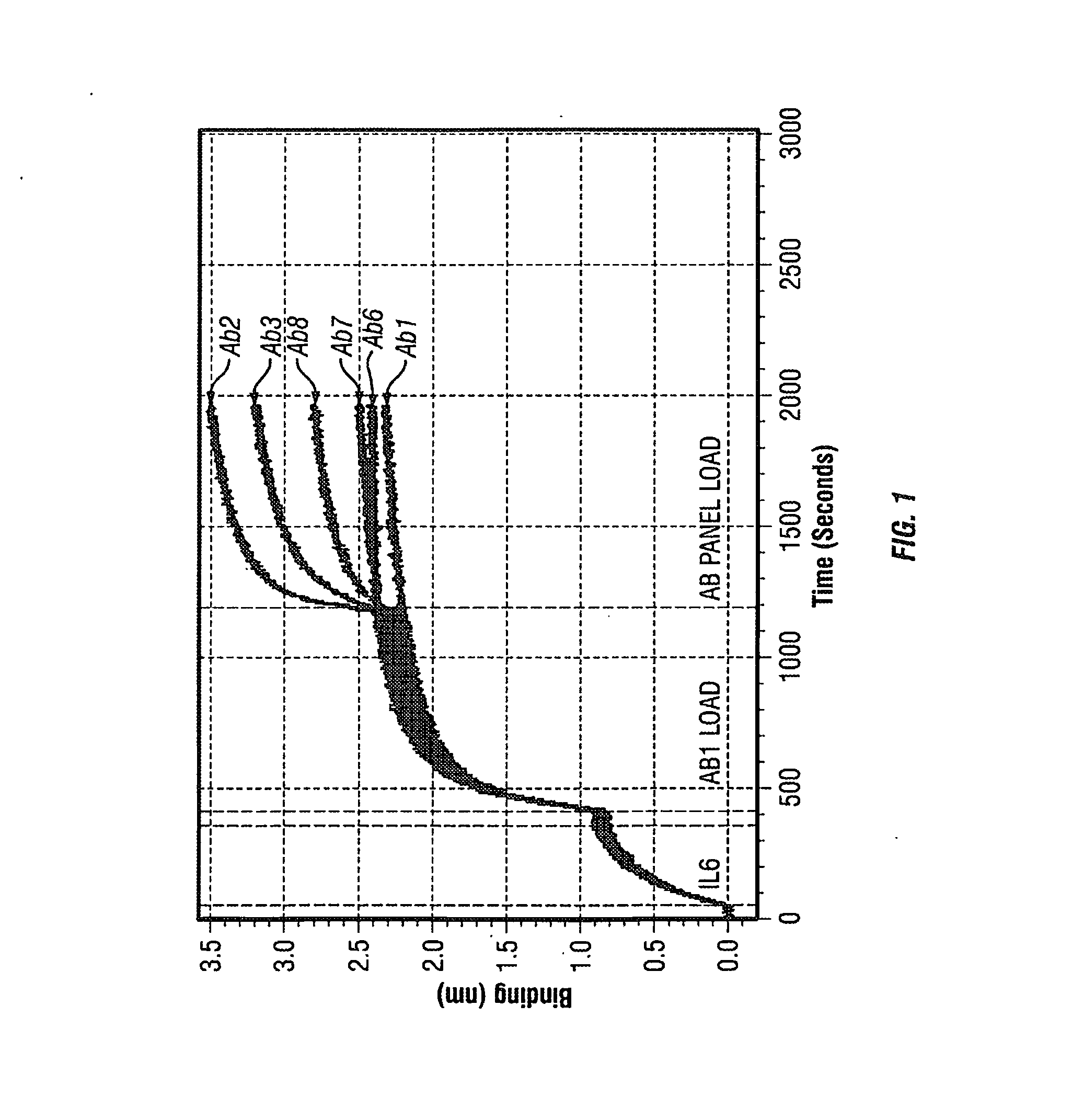
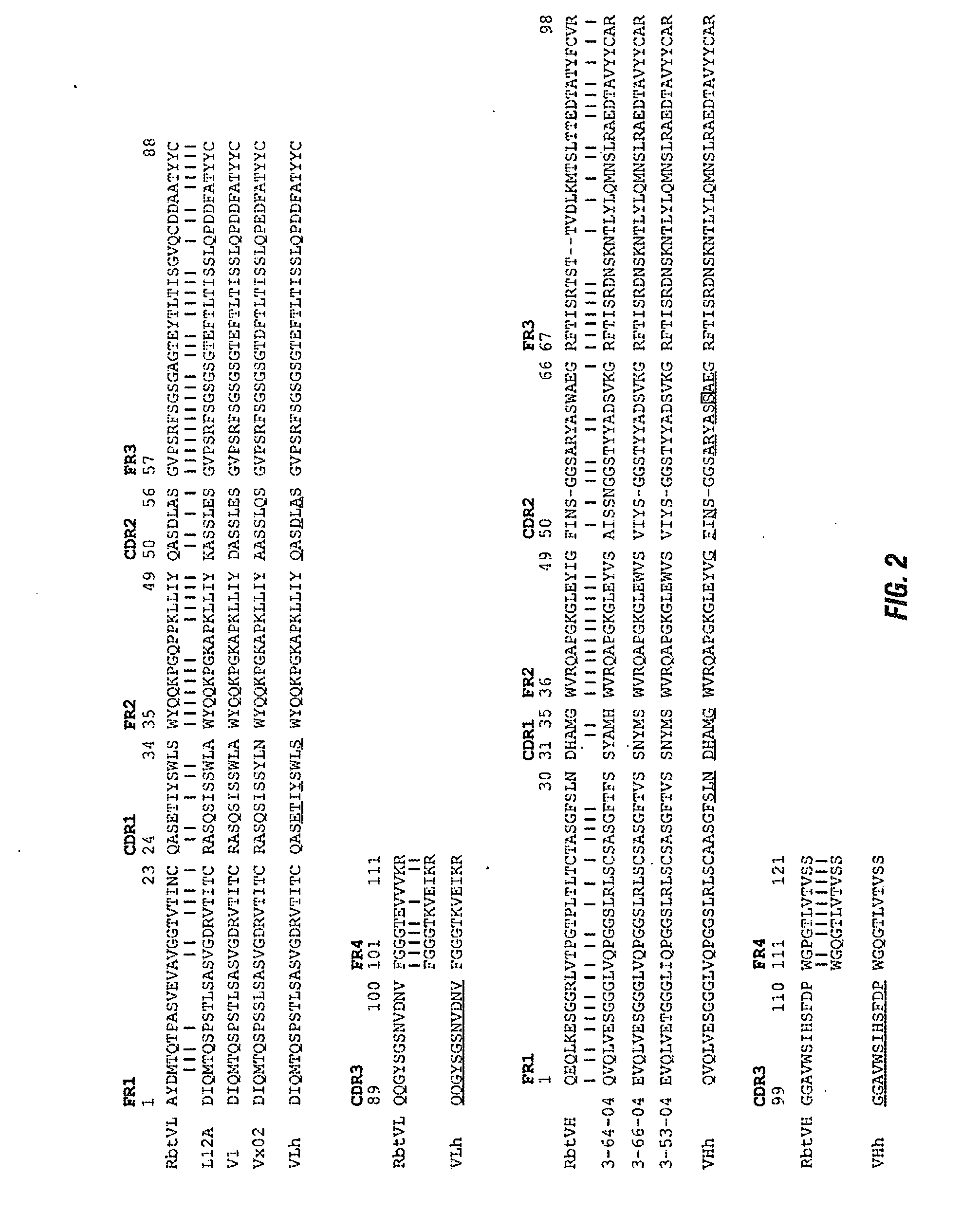
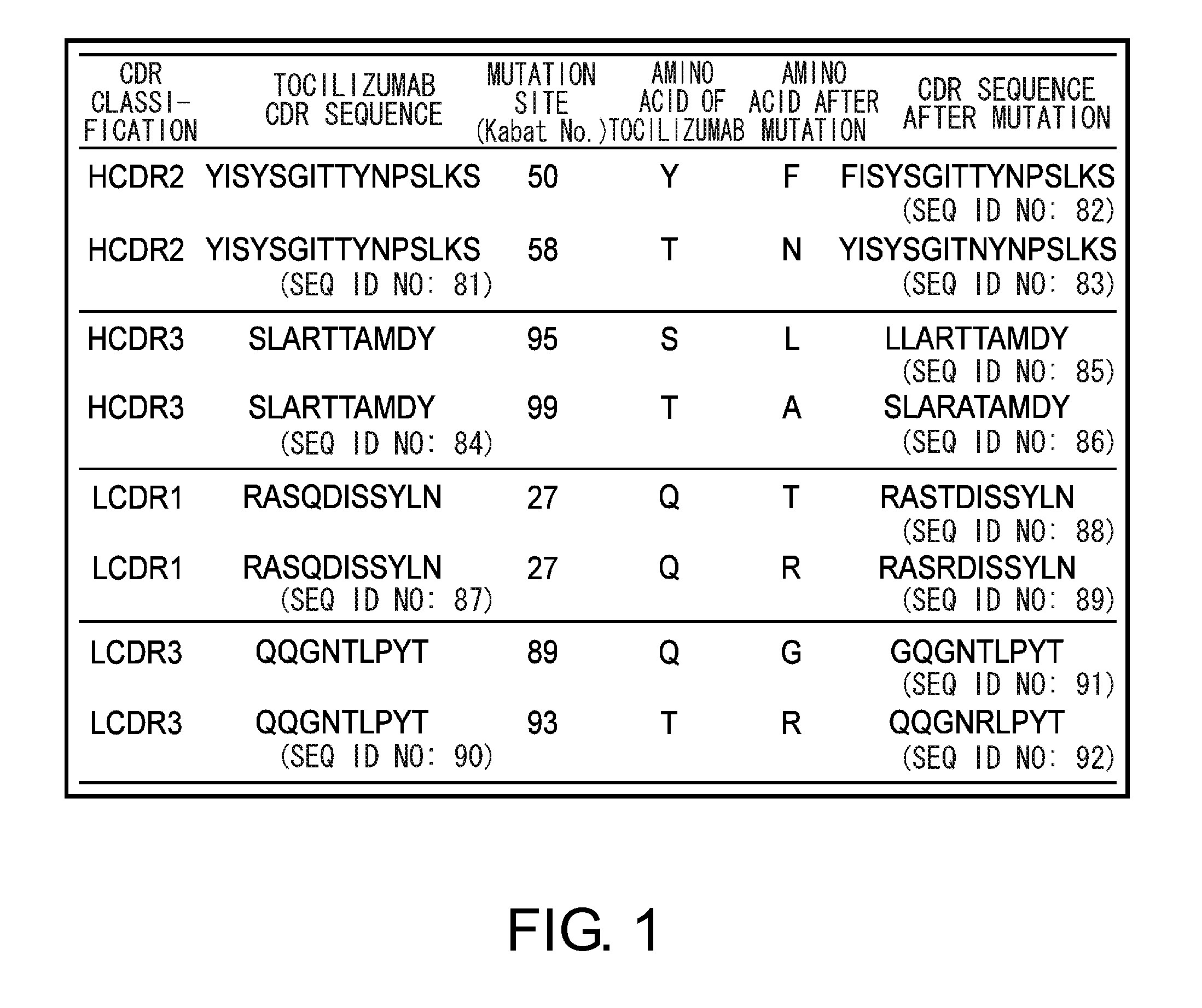
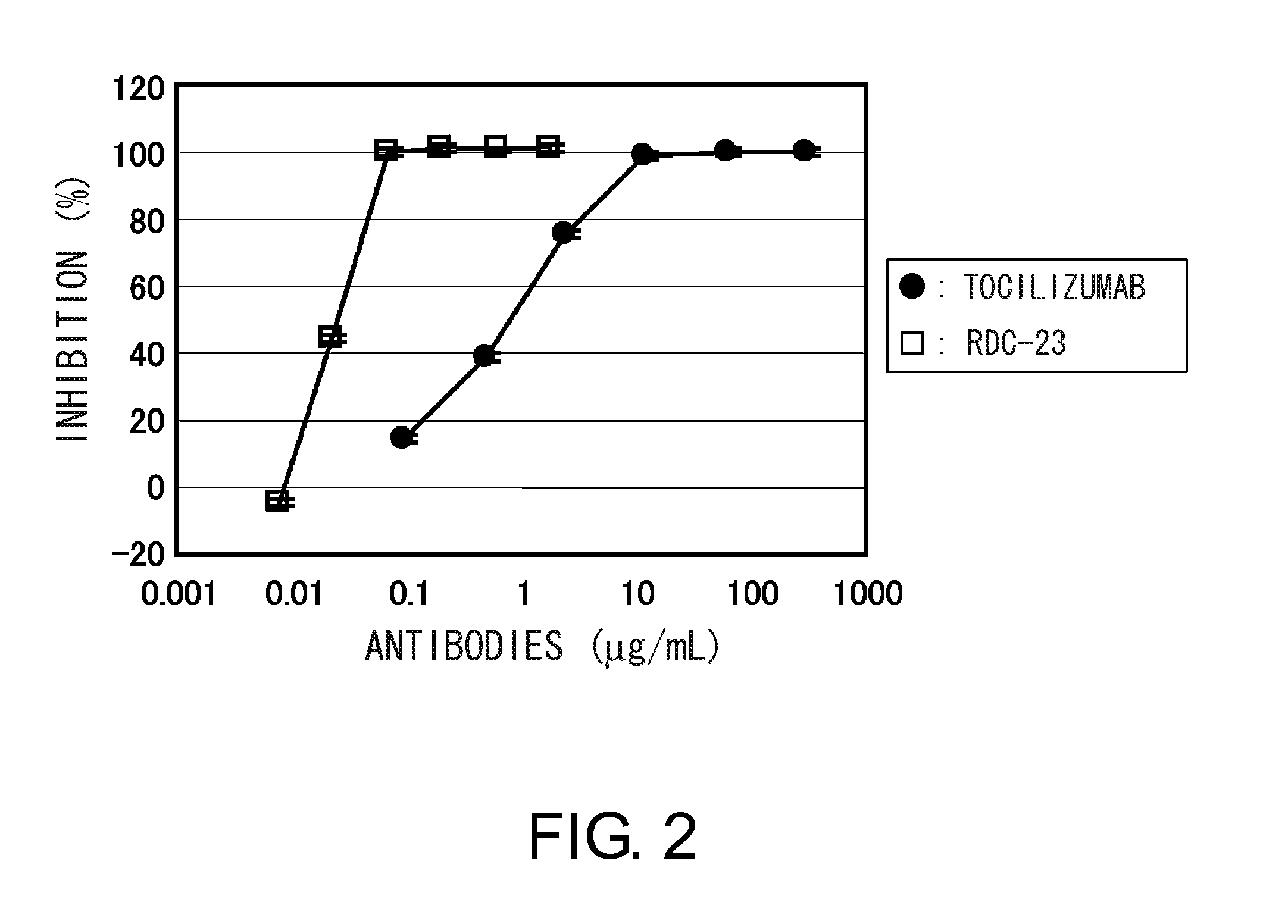
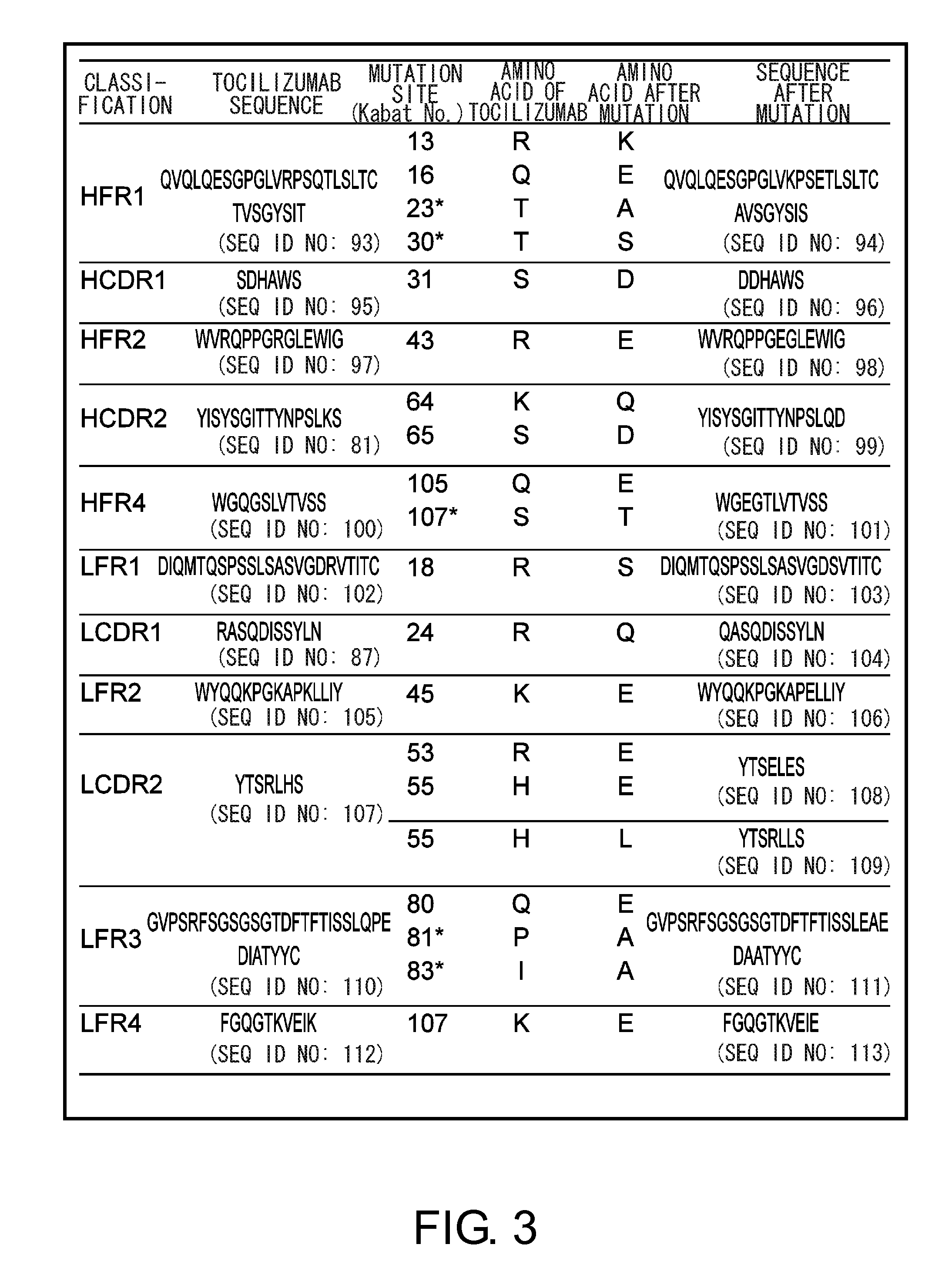
The present invention provides pharmaceutical compositions comprising second-generation molecules that are superior than TOCILIZUMAB, by altering the amino acid sequences of the variable and constant regions of TOCILIZUMAB, which is a humanized anti-IL-6 receptor IgG1 antibody, to enhance the antigen-neutralizing ability and increase the pharmacokinetics, so that the therapeutic effect is exerted with a less frequency of administration, and the immunogenicity, safety and physicochemical properties (stability and homogeneity) are improved. The present invention also provides methods for producing these pharmaceutical compositions. The present inventors have successfully generated second-generation molecules that are superior to TOCILIZUMAB by appropriately combining amino acid sequence alterations in the CDR domains, variable regions, and constant regions.
Owner:CHUGAI PHARMA CO LTD
Antagonists of il-6 to prevent or treat cachexia, weakness, fatigue and/or fever
InactiveUS20130028860A1Improve the quality of lifeAntibacterial agentsCompounds screening/testingVicrivirocVein
Owner:VITAERIS INC +1
Methods of treating anemia using anti-IL-6 antibodies
ActiveUS8337847B2Improve the quality of lifePeptide/protein ingredientsAntibody ingredientsAntibody fragmentsHemoglobin M
The invention provides methods of treating anemia in patients in need thereof, particularly in patients having anemia associated with chronic and / or inflammatory conditions such as cancers and arthritic conditions by administering antibodies or antibody fragments that bind IL-6. The treatment methods result in increased hemoglobin.
Owner:VITAERIS INC +1
Antibody molecules that bind to il-6 receptor
ActiveUS20150166666A1Good curative effectImprove pharmacokineticsSenses disorderBacteriaTocilizumabTherapeutic effect
The present invention provides pharmaceutical compositions comprising second-generation molecules that are superior than TOCILIZUMAB, by altering the amino acid sequences of the variable and constant regions of TOCILIZUMAB, which is a humanized anti-IL-6 receptor IgG1 antibody, to enhance the antigen-neutralizing ability and increase the pharmacokinetics, so that the therapeutic effect is exerted with a less frequency of administration, and the immunogenicity, safety and physicochemical properties (stability and homogeneity) are improved. The present invention also provides methods for producing these pharmaceutical compositions.The present inventors have successfully generated second-generation molecules that are superior to TOCILIZUMAB by appropriately combining amino acid sequence alterations in the CDR domains, variable regions, and constant regions.
Owner:CHUGAI PHARMA CO LTD
Agents for suppressing damage to transplanted islets after islet transplantation
ActiveUS8470316B2Reduce harmImprove survivabilityMetabolism disorderAntipyreticAcute hyperglycaemiaEphA Receptors
The present inventors investigated anti-IL-6 receptor antibodies for their effect in suppressing damage to transplanted islets after islet transplantation. As a result, they found that anti-IL-6 receptor antibodies reduced damage to transplanted islets, improved islet survival, and corrected hyperglycemia in recipients. Further, they revealed that administration of the anti-IL-6 receptor antibodies of the present invention suppressed the production of inflammatory cytokines by infiltrating cells after transplantation. Specifically, the present inventors discovered for the first time that damage to transplanted islets after islet transplantation can be suppressed by using anti-IL-6 receptor antibodies according to the present invention.
Owner:CHUGAI PHARMA CO LTD
Antibody derivatives
InactiveUS20140127209A1Improve stabilityImproved stability and half life and yieldNervous disorderHybrid immunoglobulinsAntibodyAnti-IL-6
Owner:MEDIMMUNE LTD
Human Anti-IL-6 Antibodies With Extended In Vivo Half-Life And Their Use In Treatment Of Oncology, Autoimmune Diseases And Inflammatory Diseases
InactiveUS20120034212A1Improve temperature stabilitySmall sizeFungiBacteriaAbnormal tissue growthAutoimmune responses
The present invention provides human anti-IL-6 antibodies with extended in vivo half-life. The invention further relates to pharmaceutical compositions, therapeutic compositions, and methods using therapeutic antibodies that bind to IL-6 and that has an extended in vivo half-life for the treatment and prevention of IL-6 mediated diseases and disorders, such as, but not limited to, inflammatory diseases and disorders, autoimmune diseases and disorders and tumors.
Owner:MEDIMMUNE LLC
Methods for treating a disease involving choroidal neovascularization by administering an IL-6 receptor antibody
ActiveUS8771686B2Advancement of CNV could be suppressedSenses disorderImmunoglobulins against cell receptors/antigens/surface-determinantsDiseaseNeovascularization
The present inventors focused on the fact that inflammation at the subretinal macular area enhances choroidal neovascularization, and developed pharmaceutical agents that suppress initiation or advancement of neovascularization by angiogenic factors such as VEGF. More specifically, the present inventors revealed that administering anti-IL-6 receptor monoclonal antibodies to mice treated with laser photocoagulation inhibits the development of choroidal neovascularization.
Owner:CHUGAI PHARMA CO LTD +1
Methods for suppressing acute rejection of a heart transplant
ActiveUS8623355B2Improve survivalSuppression of rejectionSurgical drugsImmunoglobulins against cell receptors/antigens/surface-determinantsHistopathology findingT cell
The effect of anti-IL-6 receptor antibodies in suppressing cytotoxic T cell induction was examined. The results showed that CTL activity against alloantigens was statistically significantly reduced in mice treated with anti-IL-6 receptor antibodies as compared to mice not treated with antibodies and mice treated with a control antibody. The anti-IL-6 receptor antibody was also administered to recipients of a mouse model for allogenic heart transplantation. As a result, histopathological findings showed that inflammatory cell infiltration into transplanted hearts was suppressed and the survival period of transplanted hearts was significantly prolonged. Thus, the present inventors for the first time discovered that administration of anti-IL-6 receptor antibodies could suppress cytotoxic T cell induction and thereby suppress acute rejection after transplantation.
Owner:CHUGAI PHARMA CO LTD +2
Subcutaneously administered anti-IL-6 receptor antibody
The present application discloses methods for treating an IL-6-mediated disorder such as rheumatoid arthritis (RA), juvenile idiopathic arthritis (JIA), systemic JIA (sJIA), polyarticular course JIA (pcJIA), systemic sclerosis, or giant cell arteritis (GCA), with subcutaneously administered antibody that binds interleukin-6 receptor (anti-IL-6R antibody). In particular, it relates to identification of a fixed dose of anti-IL-6R antibody, e.g. tocilizumab, which is safe and effective for subcutaneous administration in patients with IL-6-mediated disorders. In addition, formulations and devices useful for subcutaneous administration of an anti-IL-6R antibody are disclosed.
Owner:CHUGAI PHARMA CO LTD
Methods for treating myocardial infarction comprising administering an IL-6 inhibitor
ActiveUS8945558B2Improve the situationSuppressing left ventricular remodelingPeptide/protein ingredientsImmunoglobulins against cytokines/lymphokines/interferonsCardiac echoLeft ventricular size
The present inventors investigated the effects of anti-IL-6 receptor antibodies on improving the condition of infarcted areas in myocardial infarction, and on suppressing left ventricular remodeling after myocardial infarction. As a result, the administration of anti-IL-6 receptor antibodies significantly suppressed the increase of MPO activity in the infarcted area and suppressed myocardial MCP-1 expression in both the infarcted area and the non-infarcted area. Furthermore, echocardiography and histological examinations revealed that cardiac hypertrophy is also suppressed.
Owner:CHUGAI PHARMA CO LTD
Anti-IL-6 monoclonal antibodies
The present invention provides novel monoclonal antibodies that bind specifically to IL-6. The antibodies of the invention comprise a variable heavy chain (VH) region selected from any of the VH regions disclosed herein as well as amino acid variants thereof, and / or a variable light chain (VL) region selected from any of the VL regions disclosed herein as well as amino acid variants thereof. The invention also provides methods of treating diseases and disorders associated with IL-6 expression and / or activity.
Owner:VACCINEX
Treatment of inflammatory, non-infectious, autoimmune, vasculitic, degenerative vascular, host-v-graft diseases, Alzheimers disease, and amyloidosis using mammalian, dsDNA vaccination
The present invention relates generally to compositions and methods using mammalian, dsDNA (Double Stranded Deoxyribonucleic Acid) vaccination for the induction and maintenance of regulator suppressor T cells resulting in suppression of non infectious, and post infectious, inflammatory, allergic, auto-immune, vasculitic, certain degenerative vascular, and graft versus host diseases, with or without the use of IL-10, and with or without the use or TGFβ, with or without the use of anti-IL 6 receptor antibody, anti TNF antibody and or Plasmapheresis, IVIG, Corticosteroids, Methotrexate, Bromocriptine, and or vitamin D analogues.
Owner:LAWLESS OLIVER J
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com