Patents
Literature
96 results about "Stenotic lesion" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Medical Definition of stenotic. : of, relating to, characterized by, or causing stenosis. stenotic lesions.
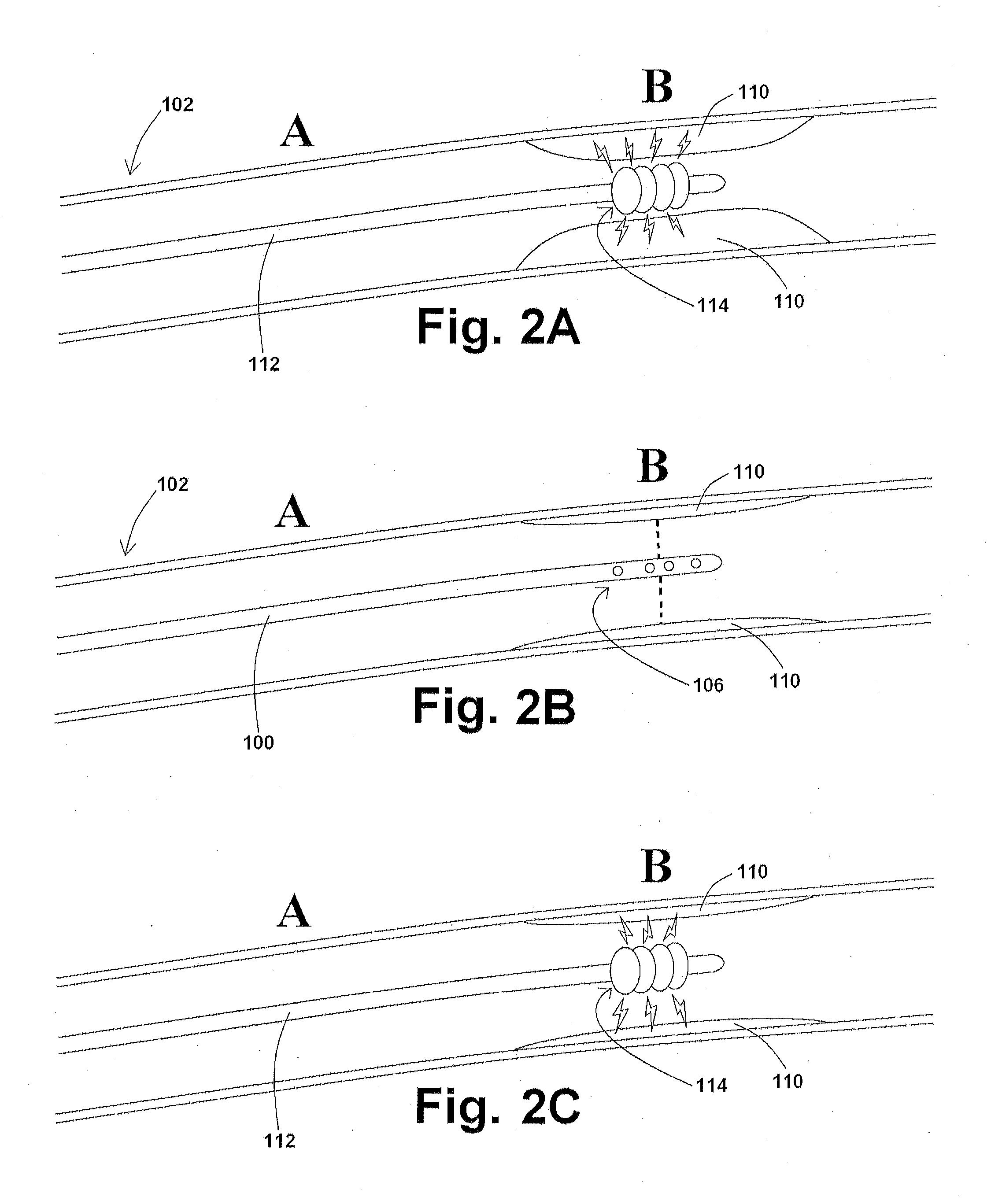
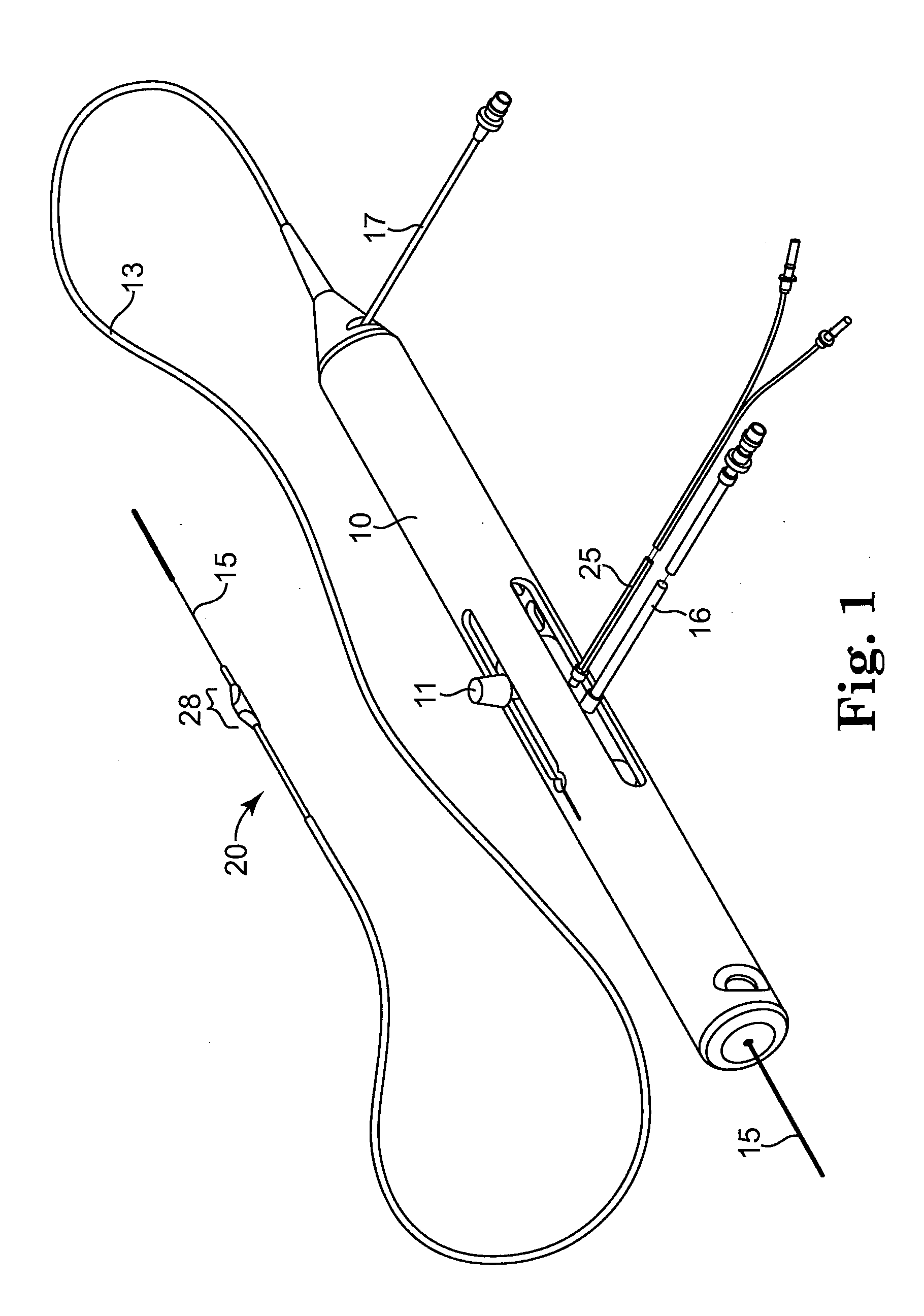
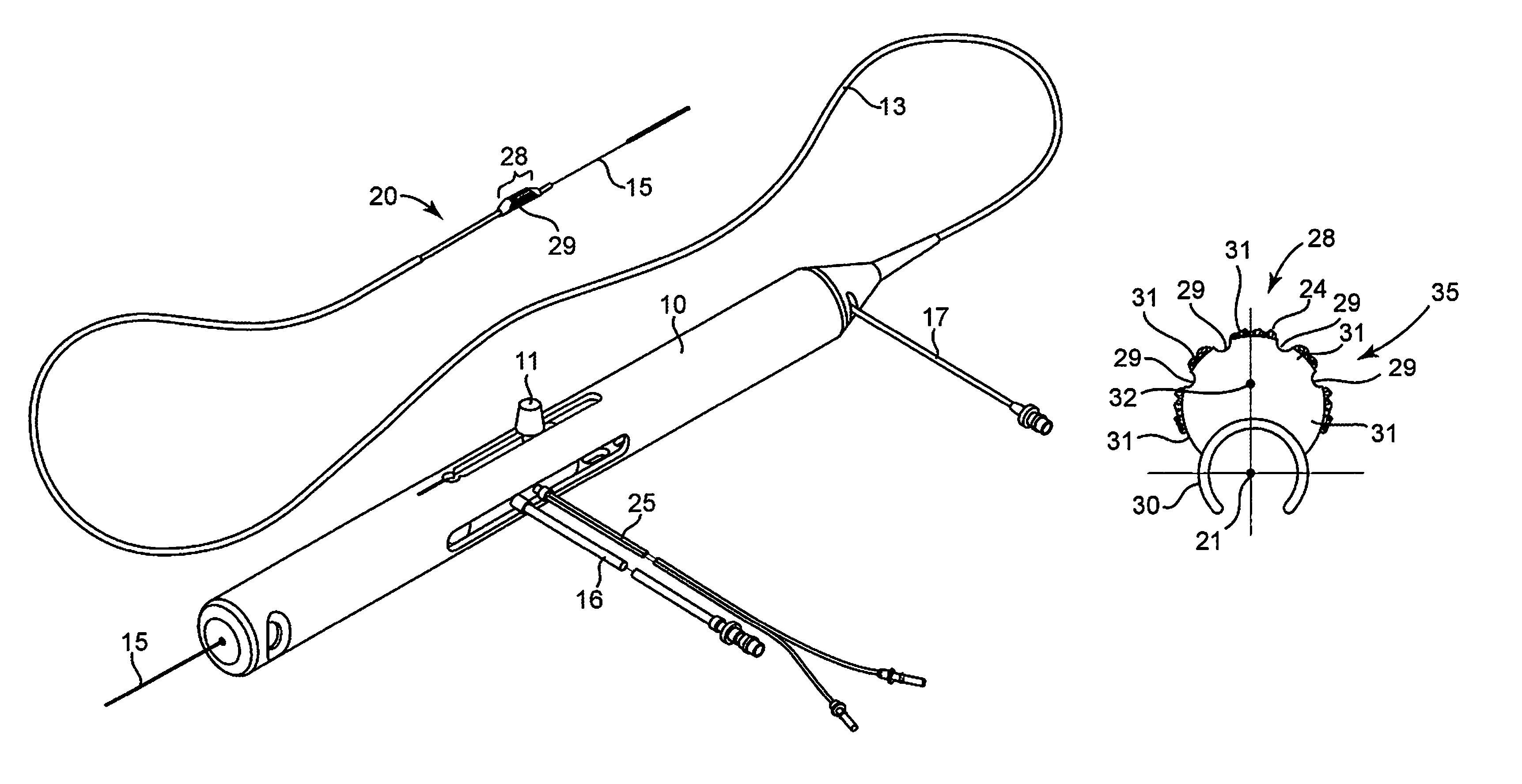
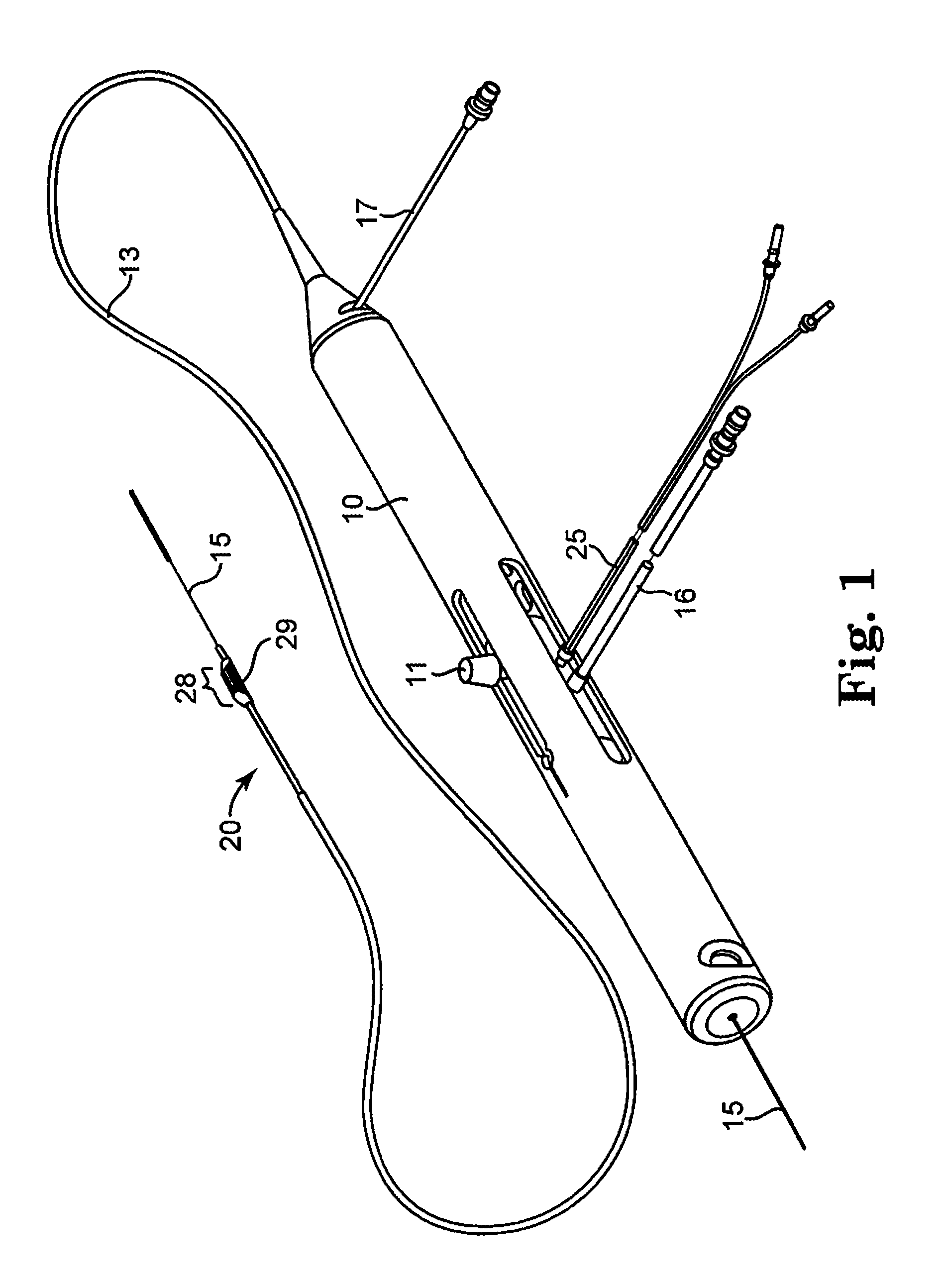
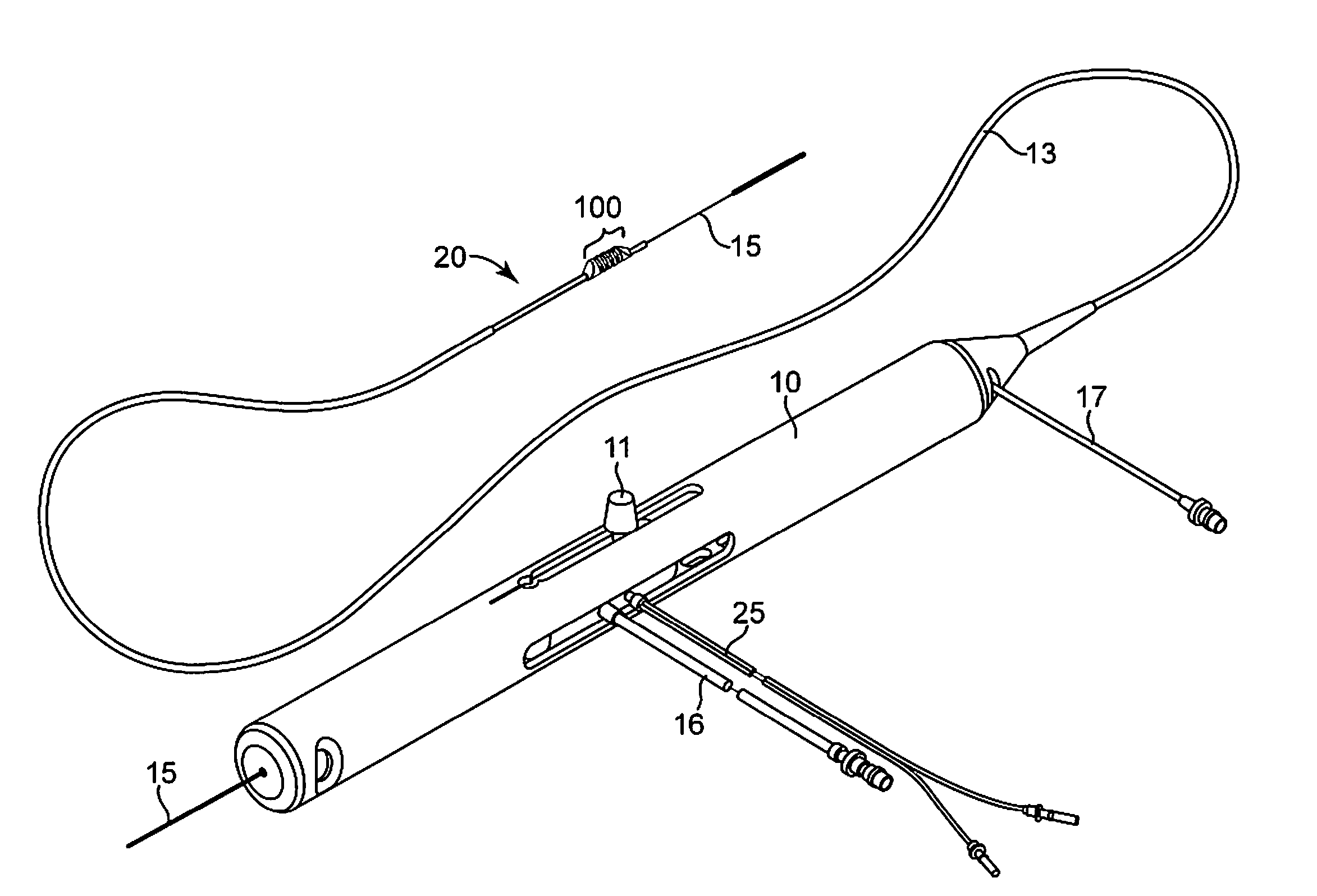
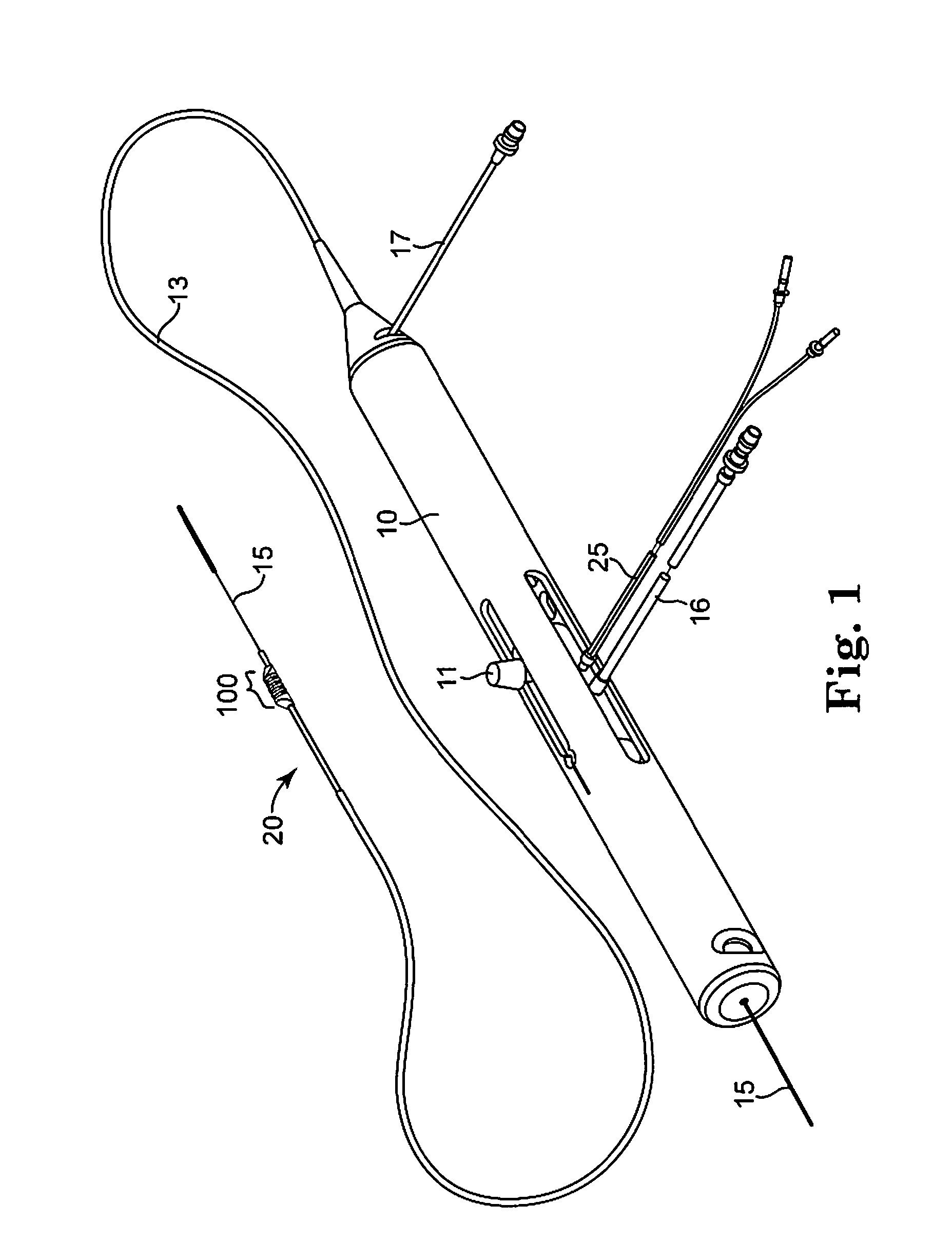
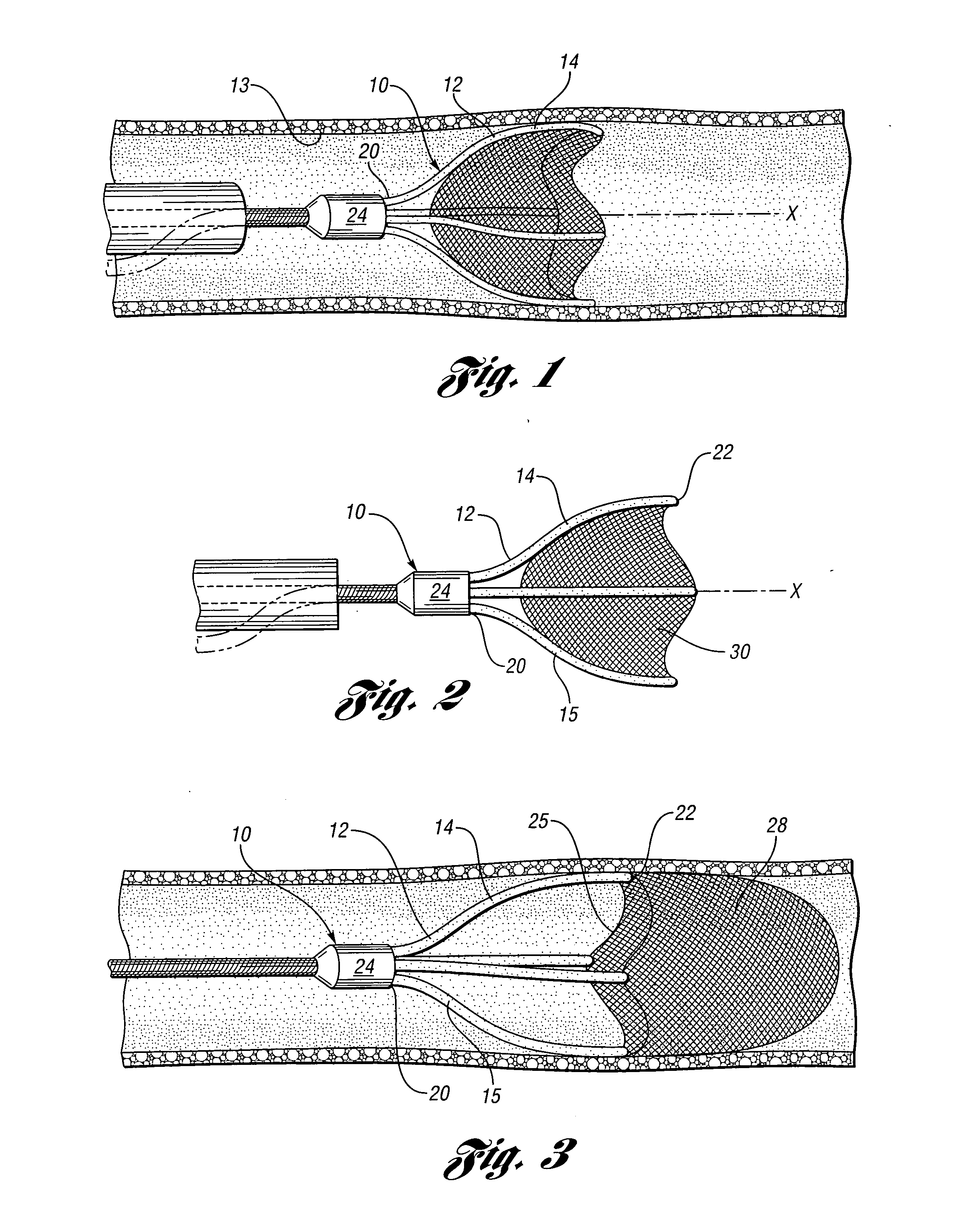
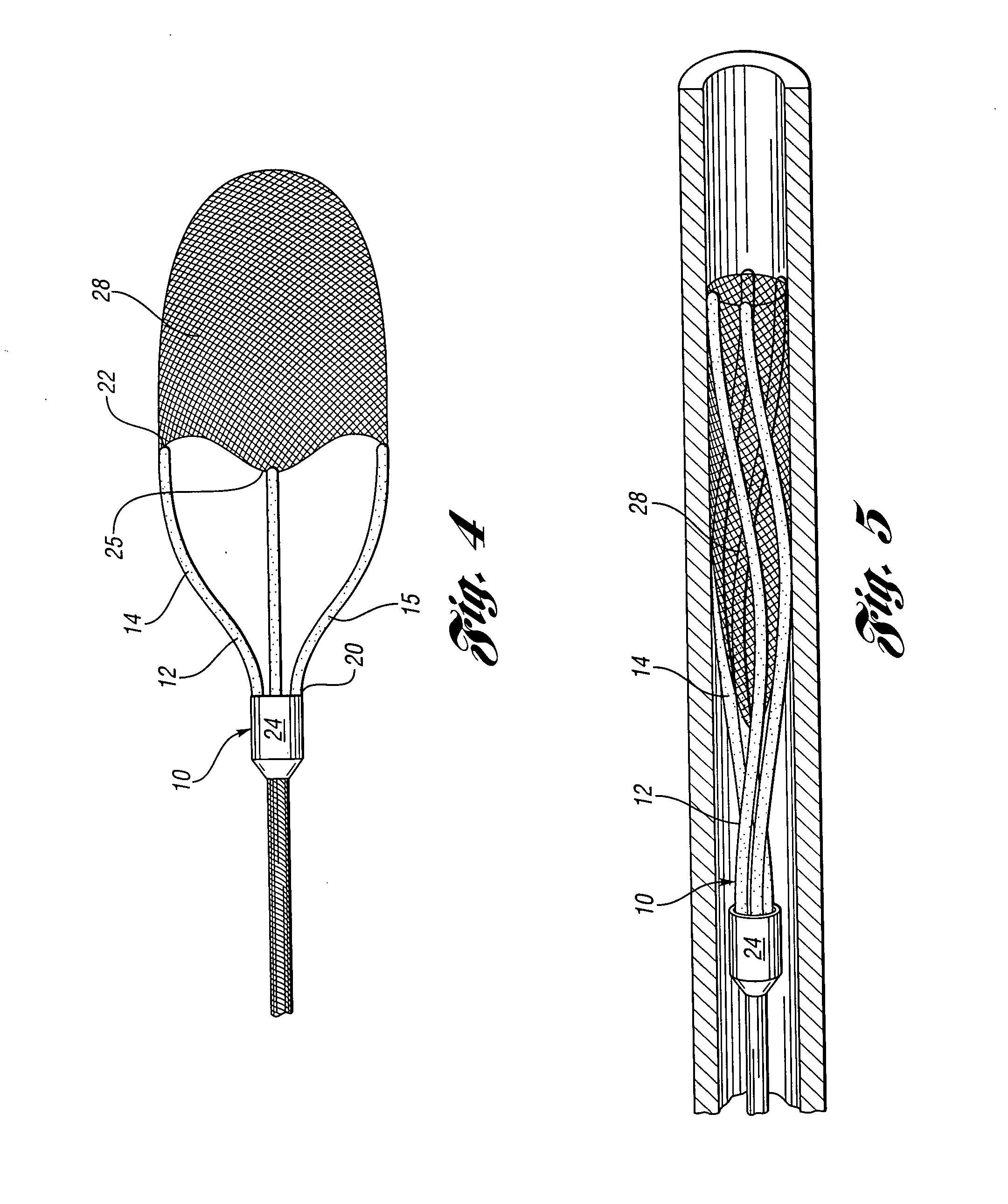
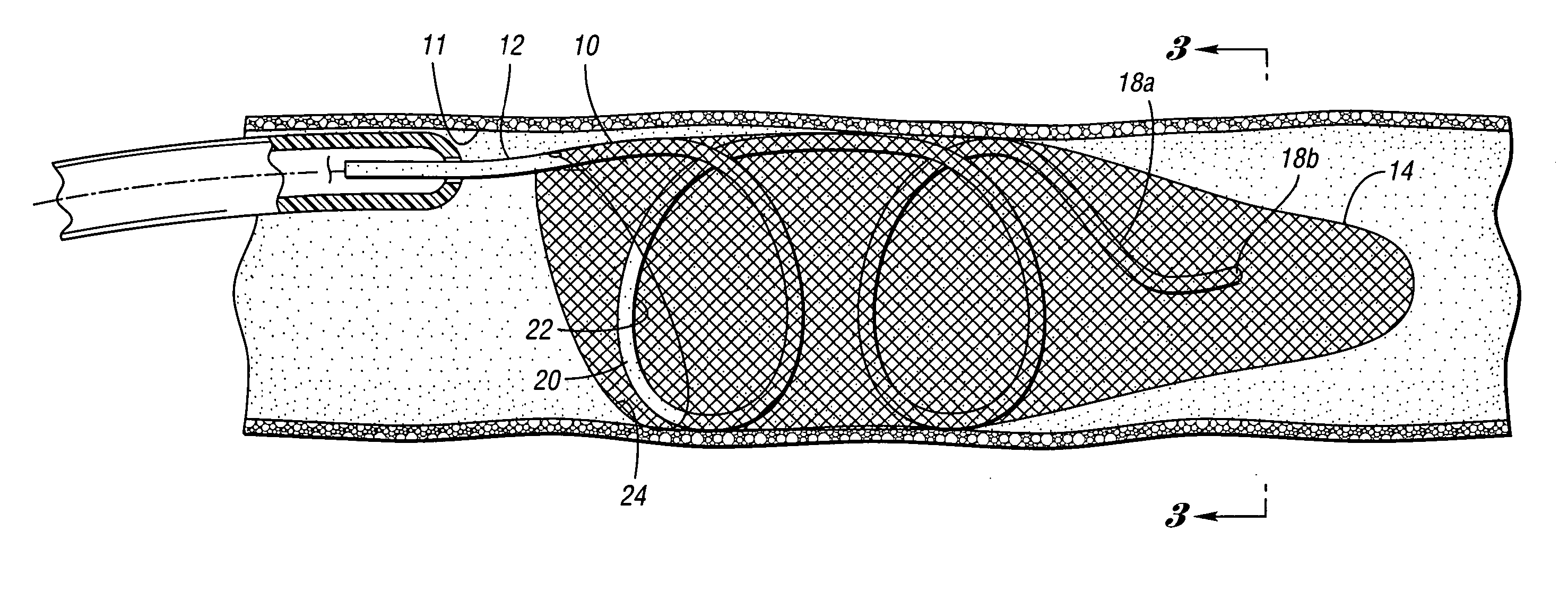
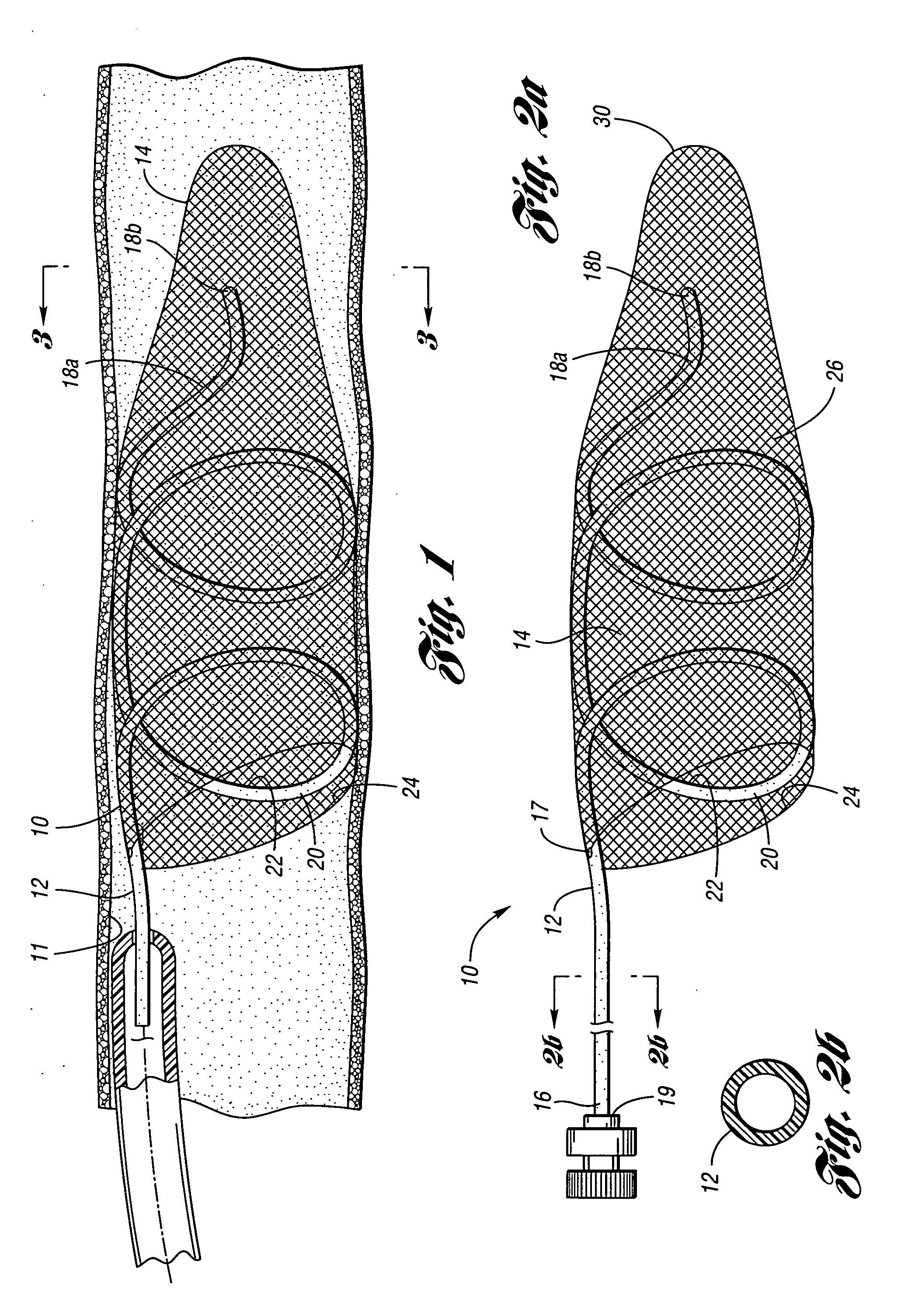
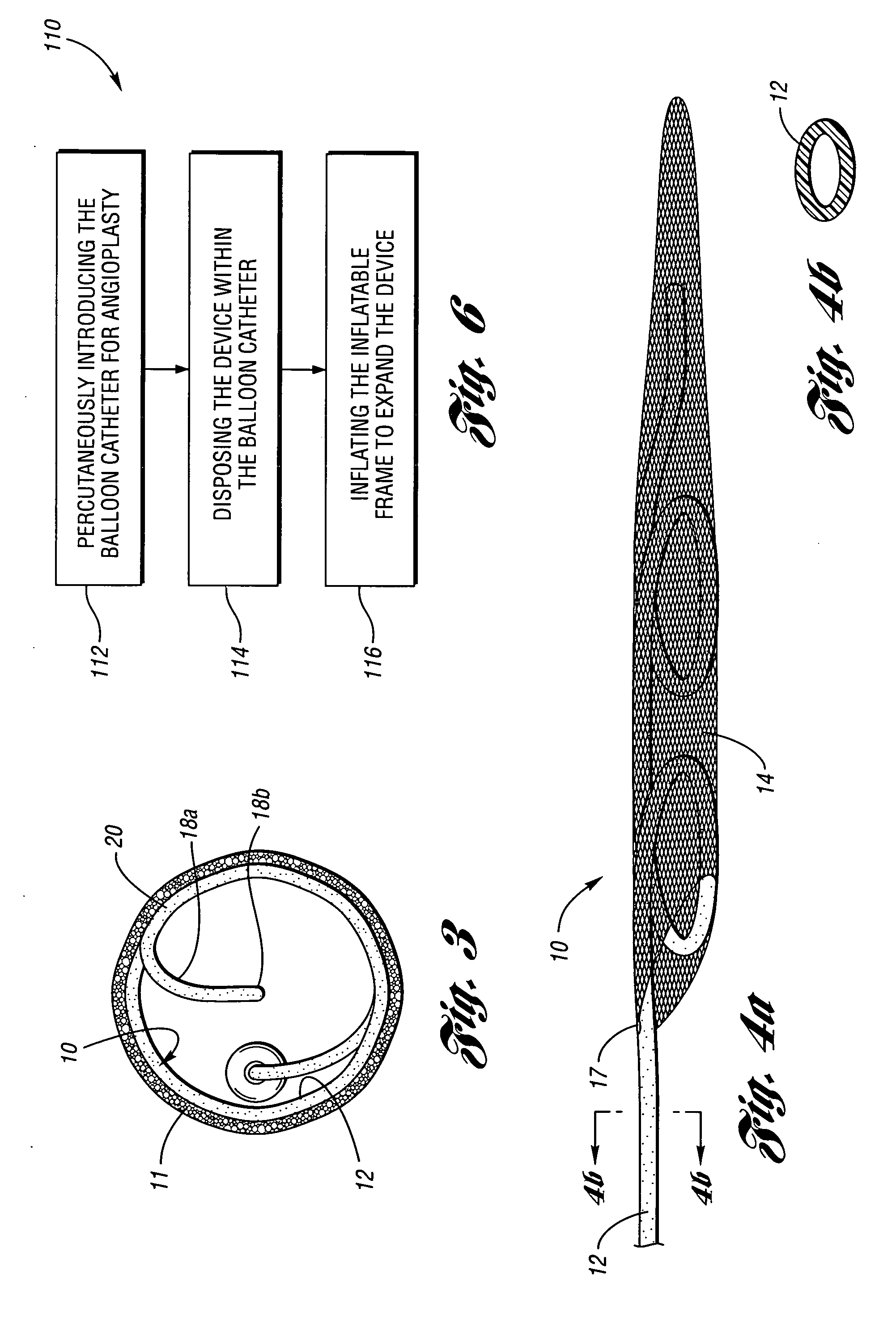
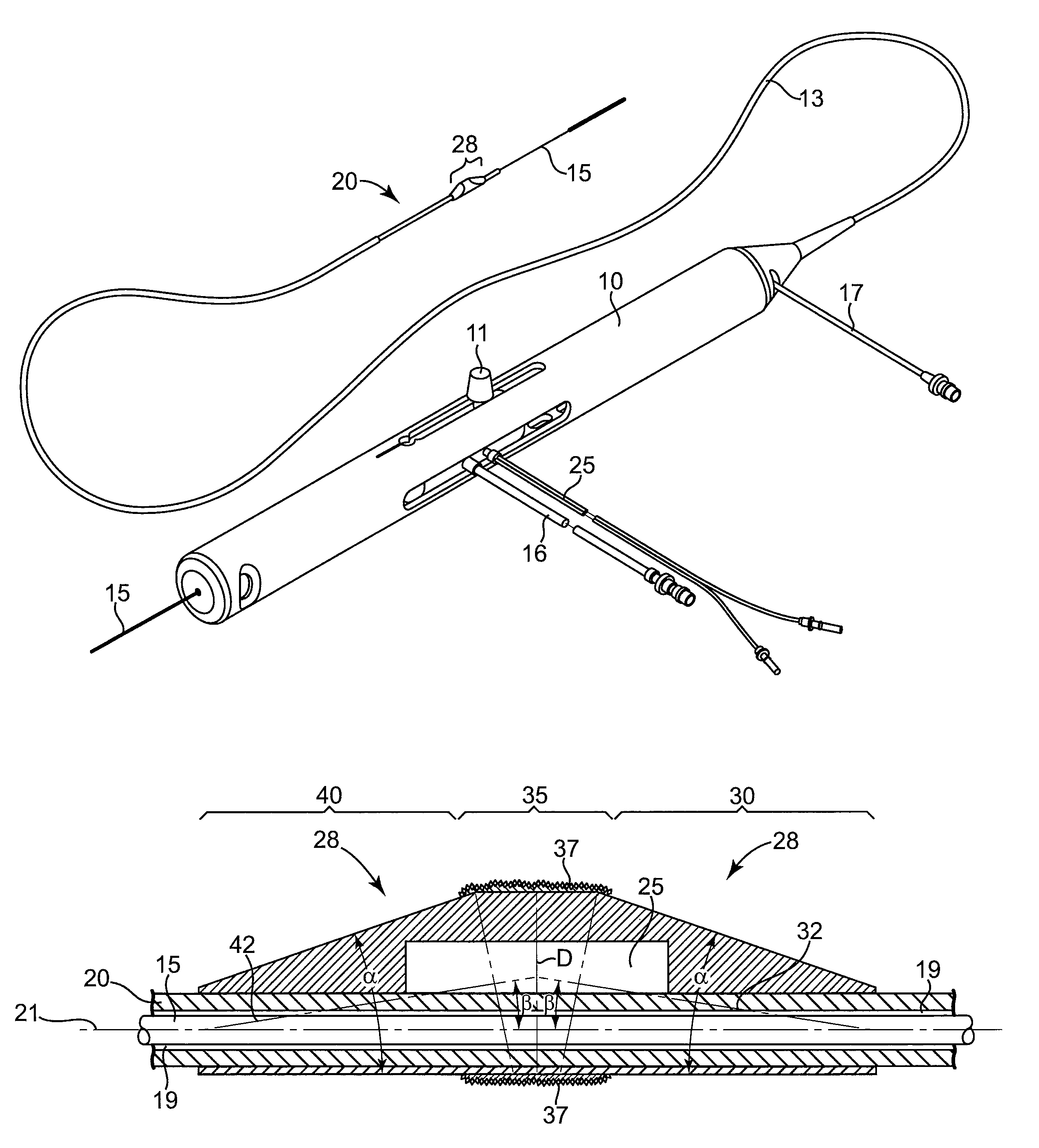
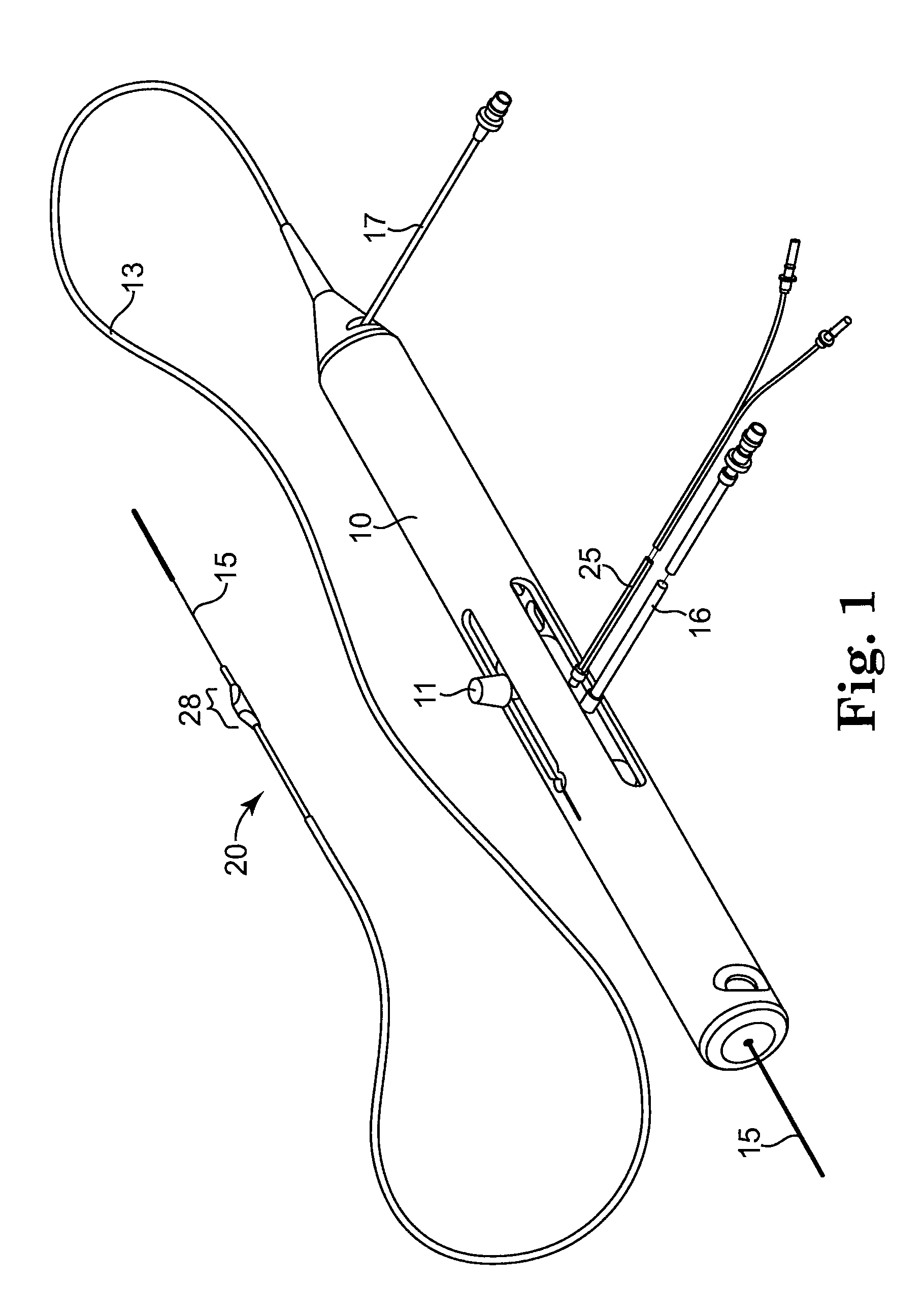
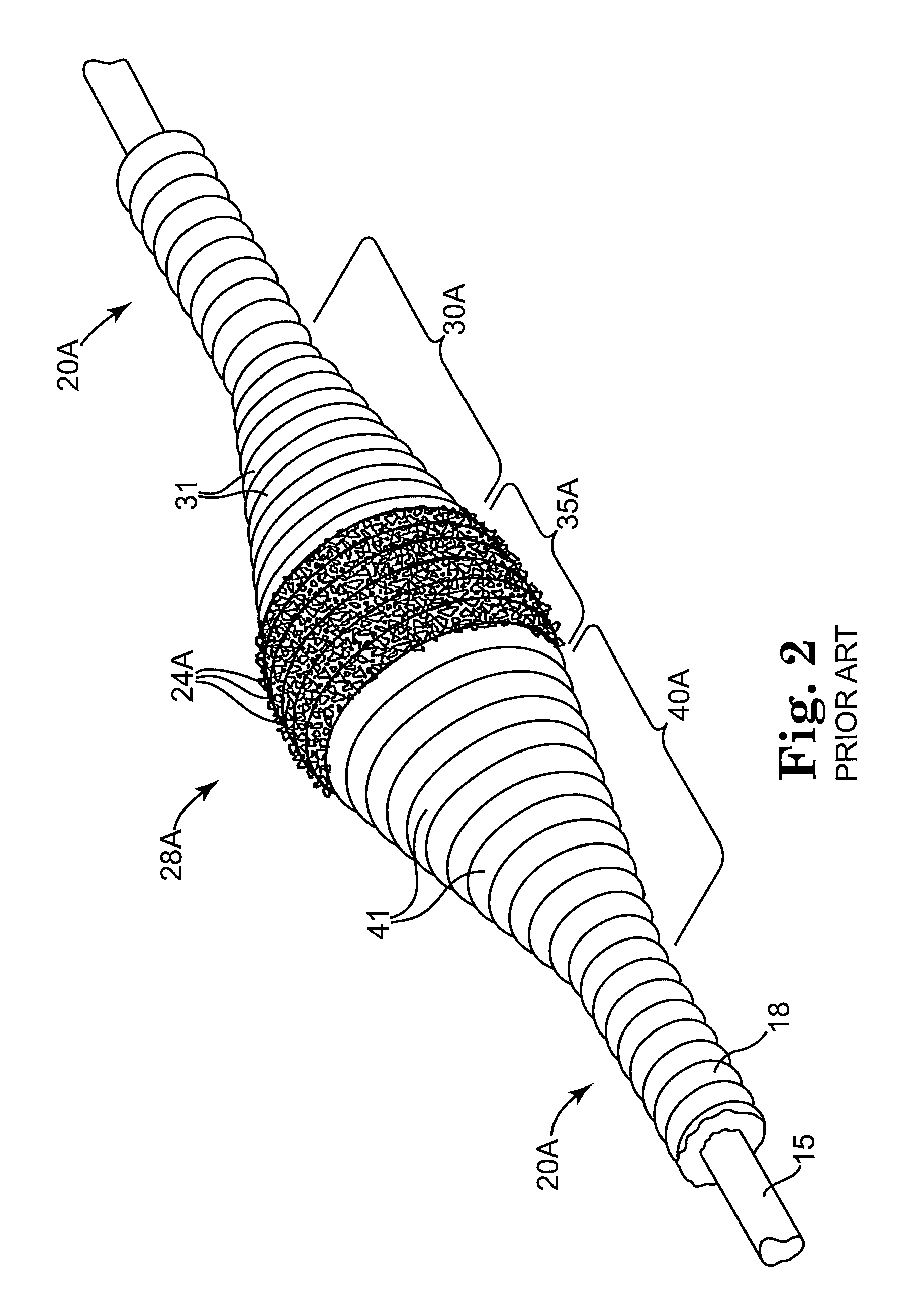
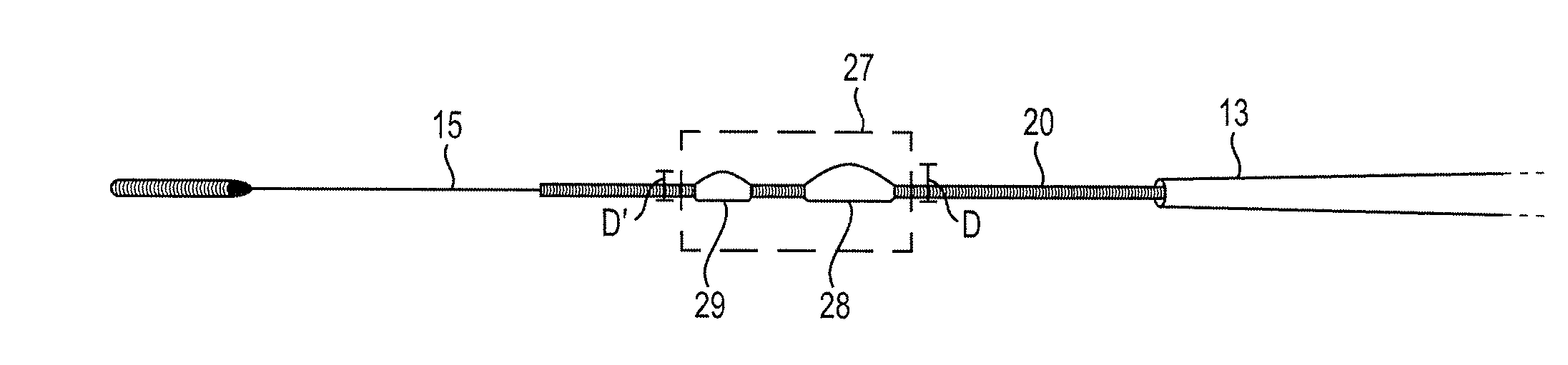
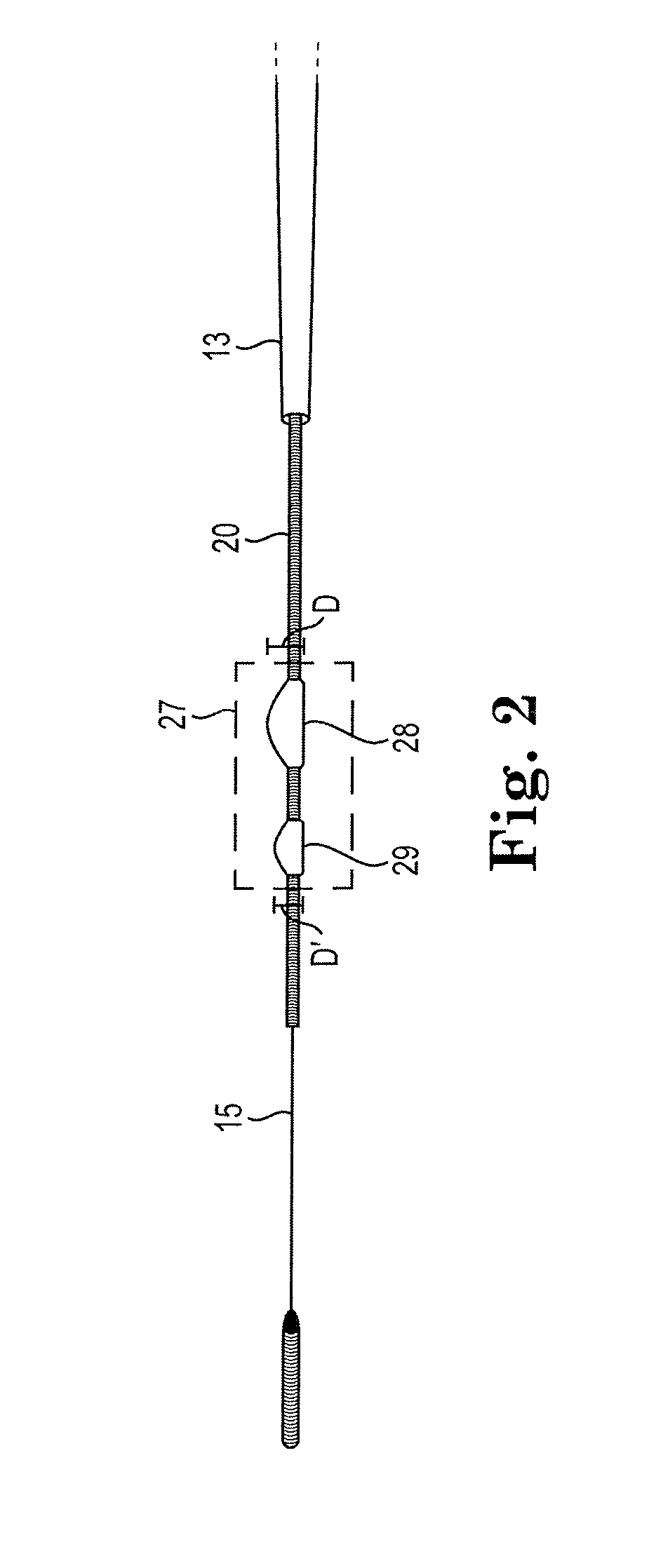
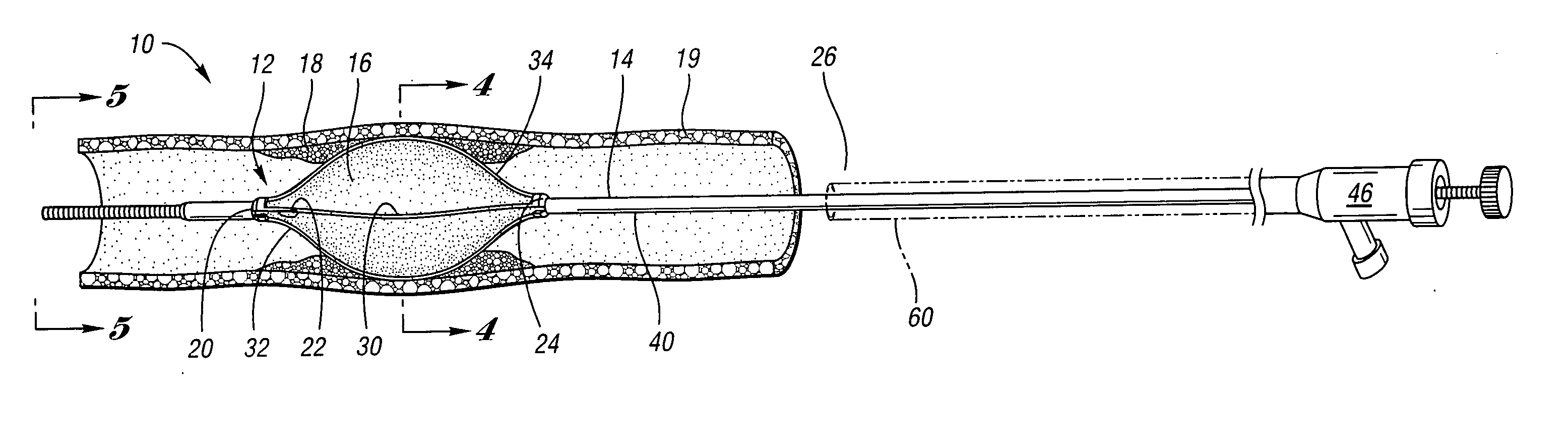
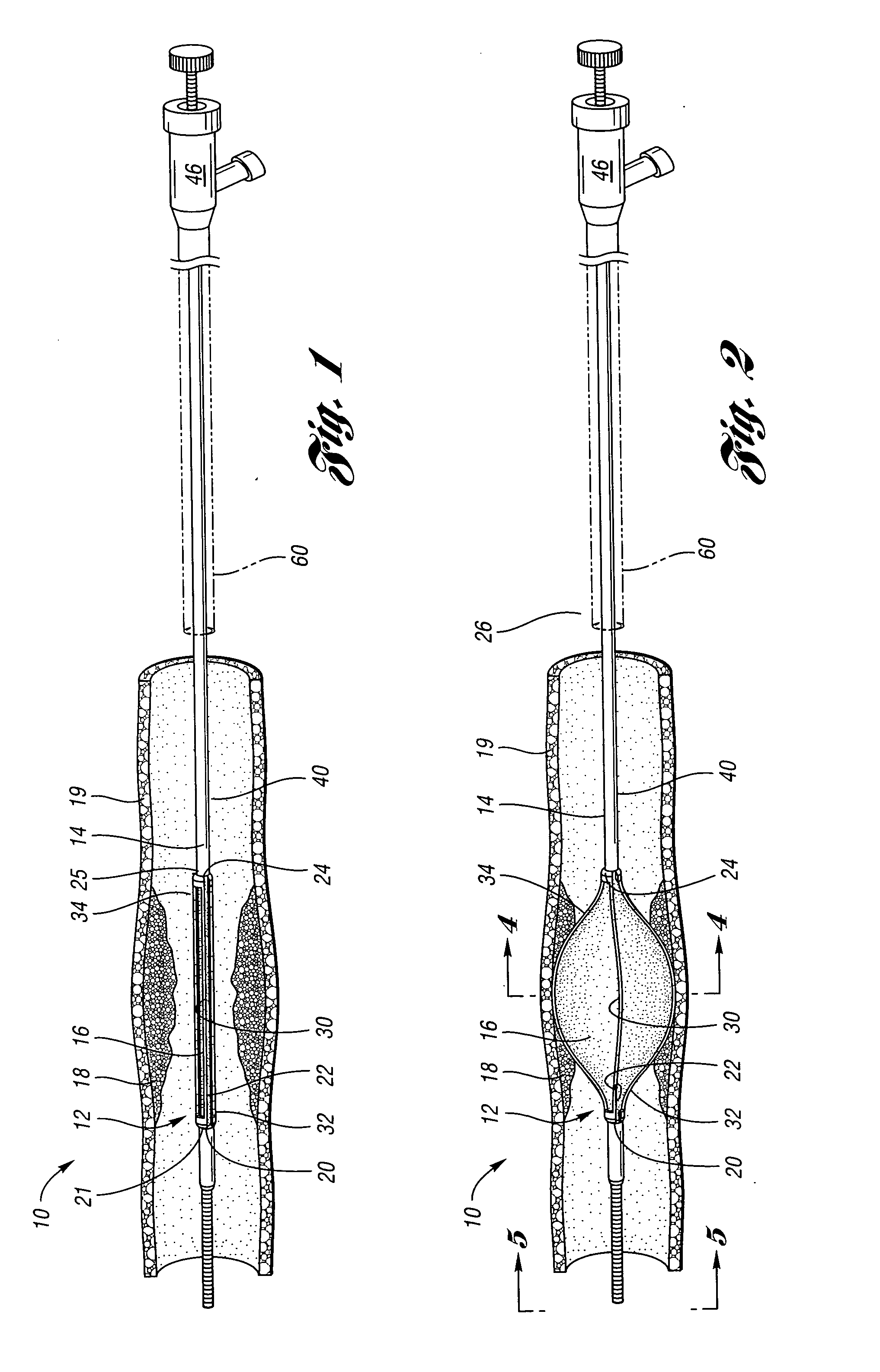
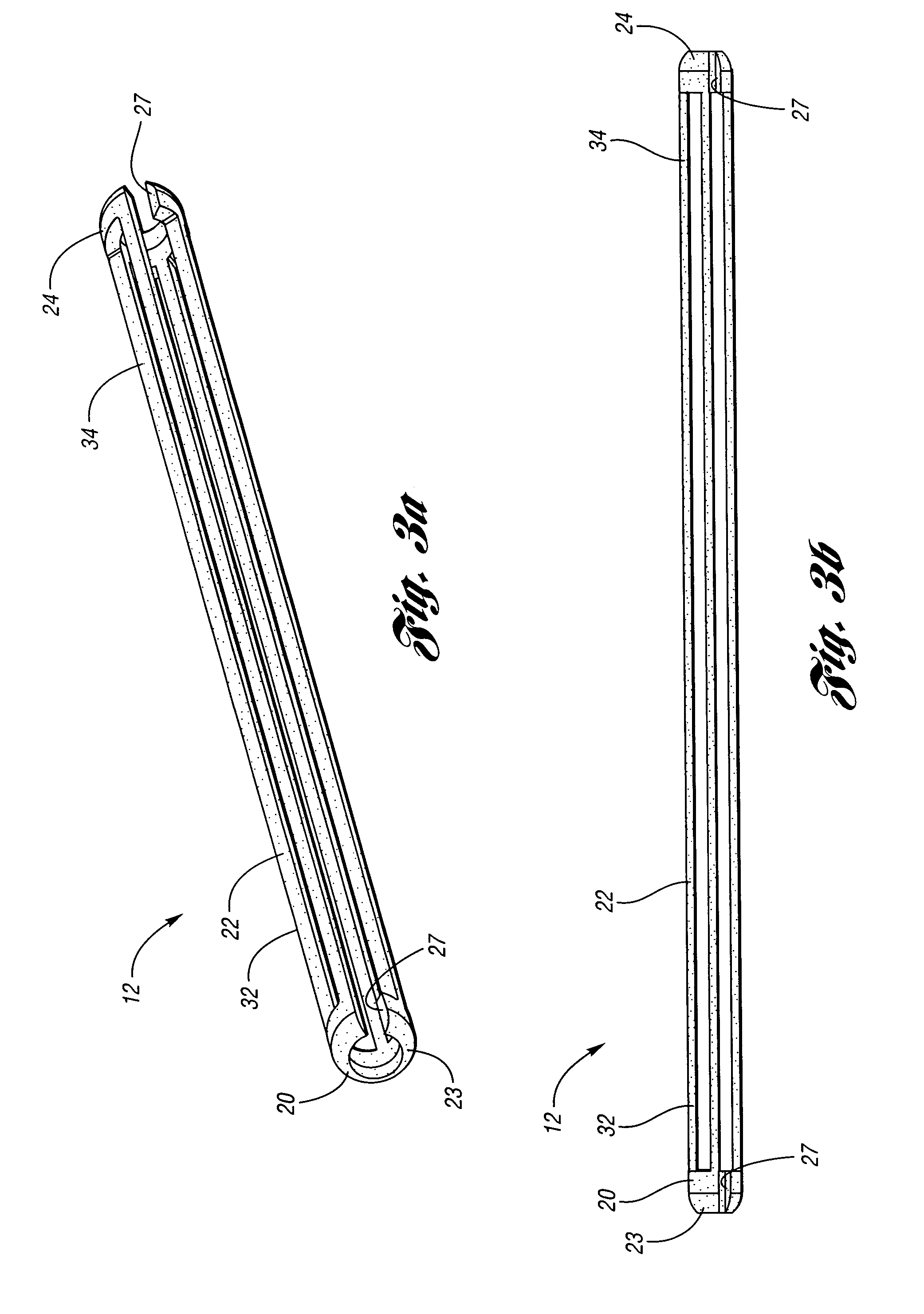
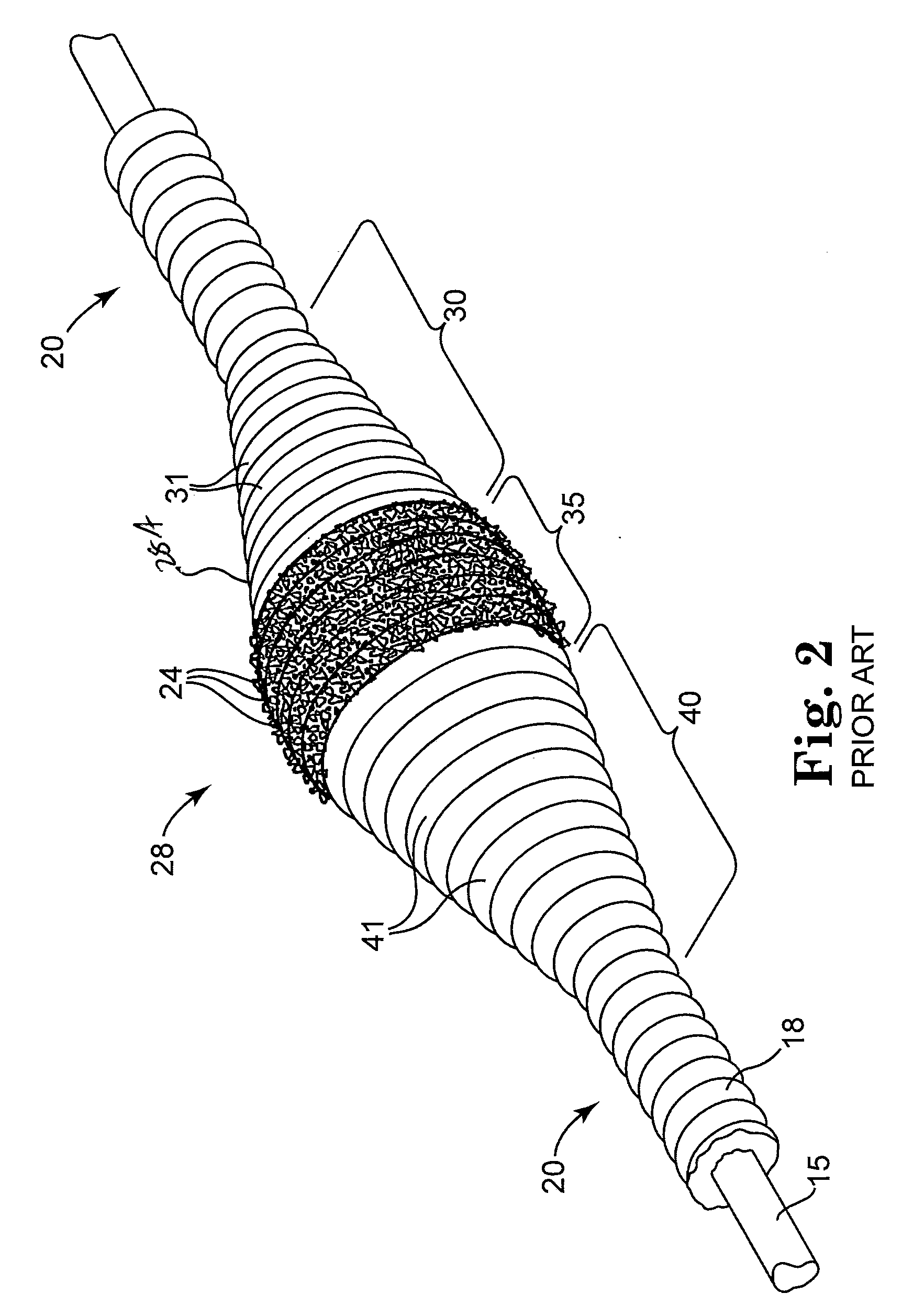
Devices and methods for controlling expandable prostheses during deployment
InactiveUS20050288766A1Reduce deliveryPrevents excessive spacing and overlapStentsBlood vesselsStenotic lesionProsthesis
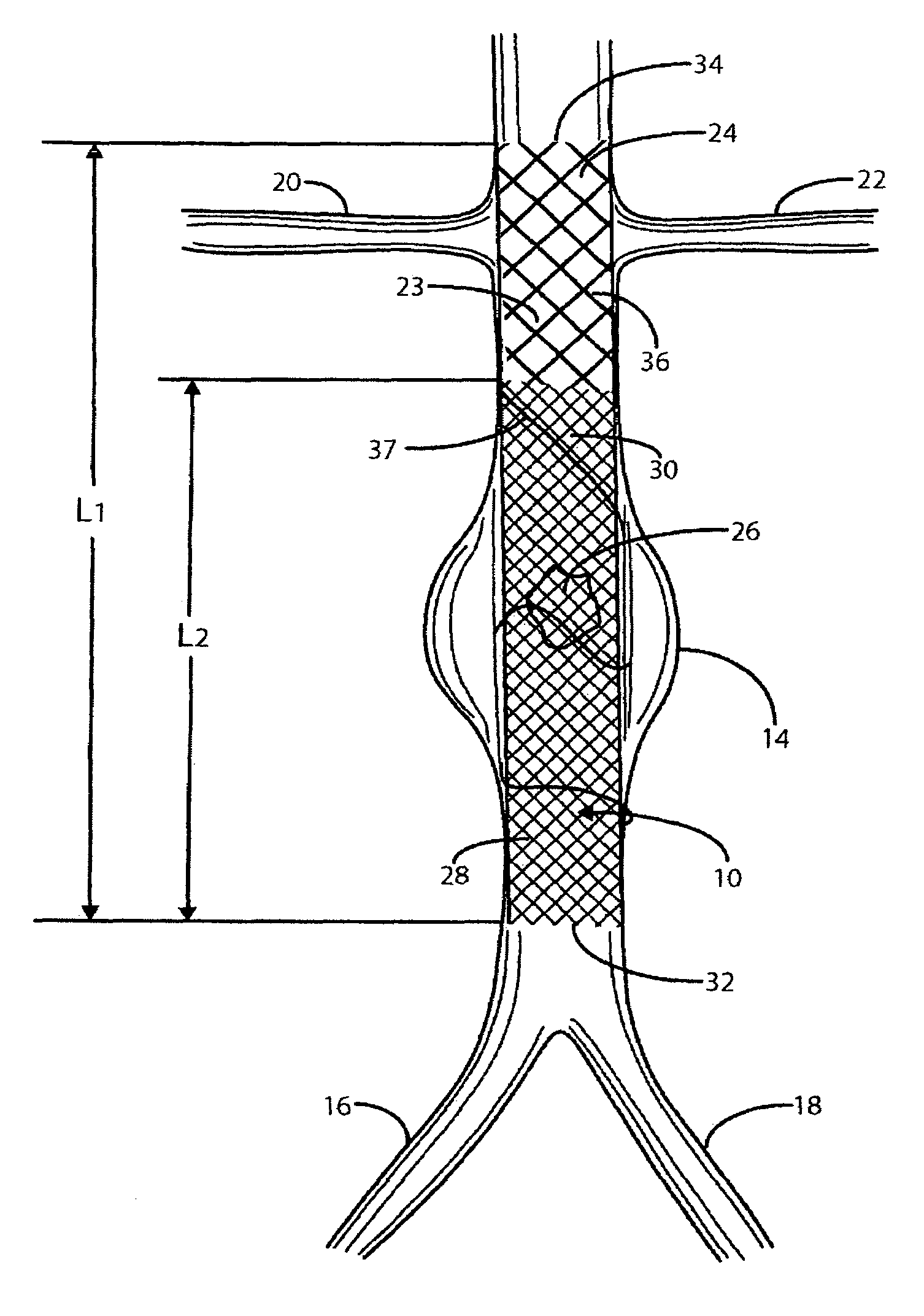
Prosthesis delivery devices and methods are provided that enable precise control of prosthesis position during deployment. The prosthesis delivery devices may carry multiple prostheses and include deployment mechanisms for delivery of a selectable number of prostheses. Control mechanisms are provided in the prosthesis delivery devices that control either or both of the axial and rotational positions of the prostheses during deployment. This enables the deployment of multiple prostheses at a target site with precision and predictability, eliminating excessive spacing or overlap between prostheses. In particular embodiments, the prostheses of the invention are deployed in stenotic lesions in coronary or peripheral arteries or in other vascular locations.
Owner:XTENT INC
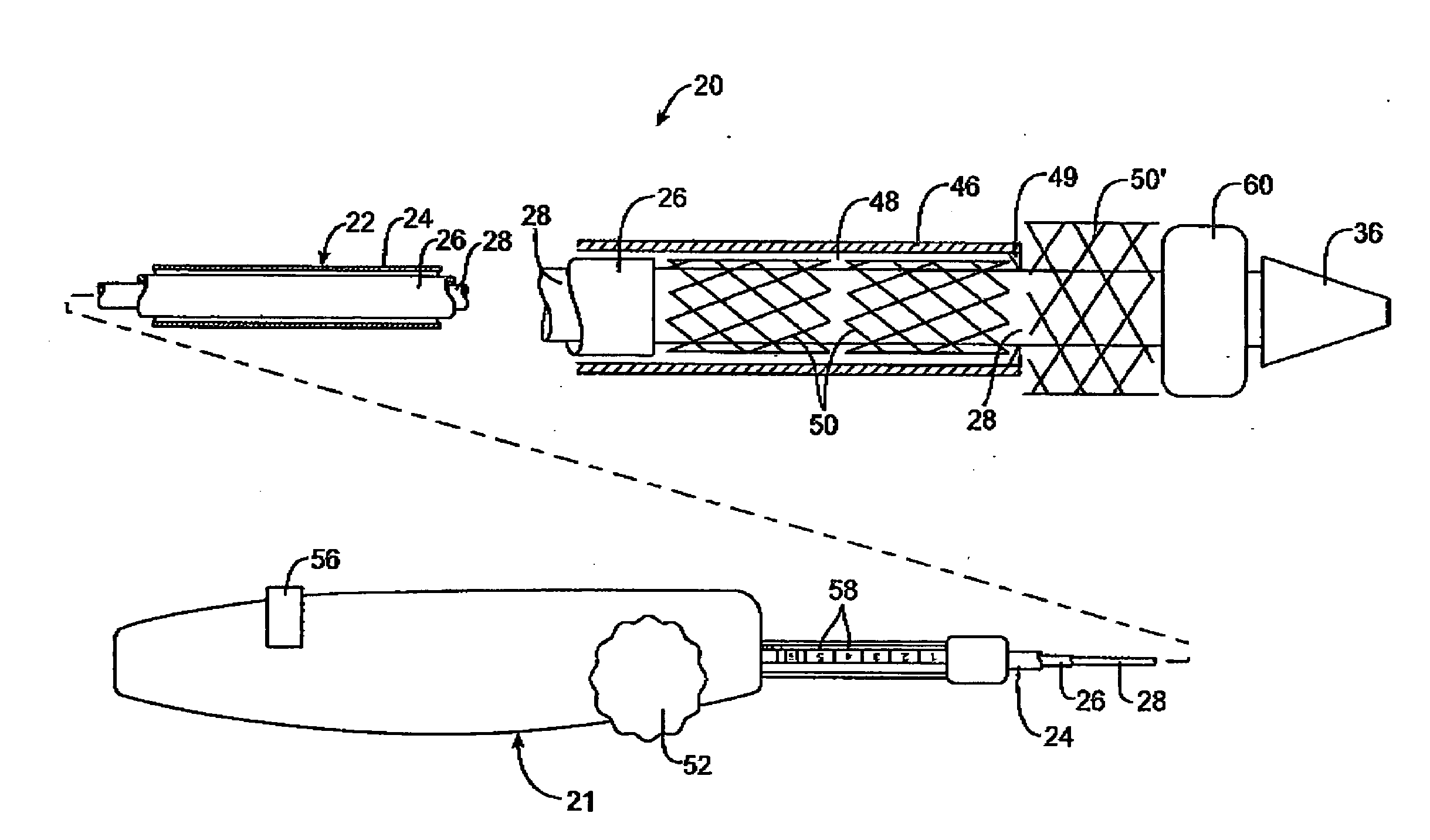
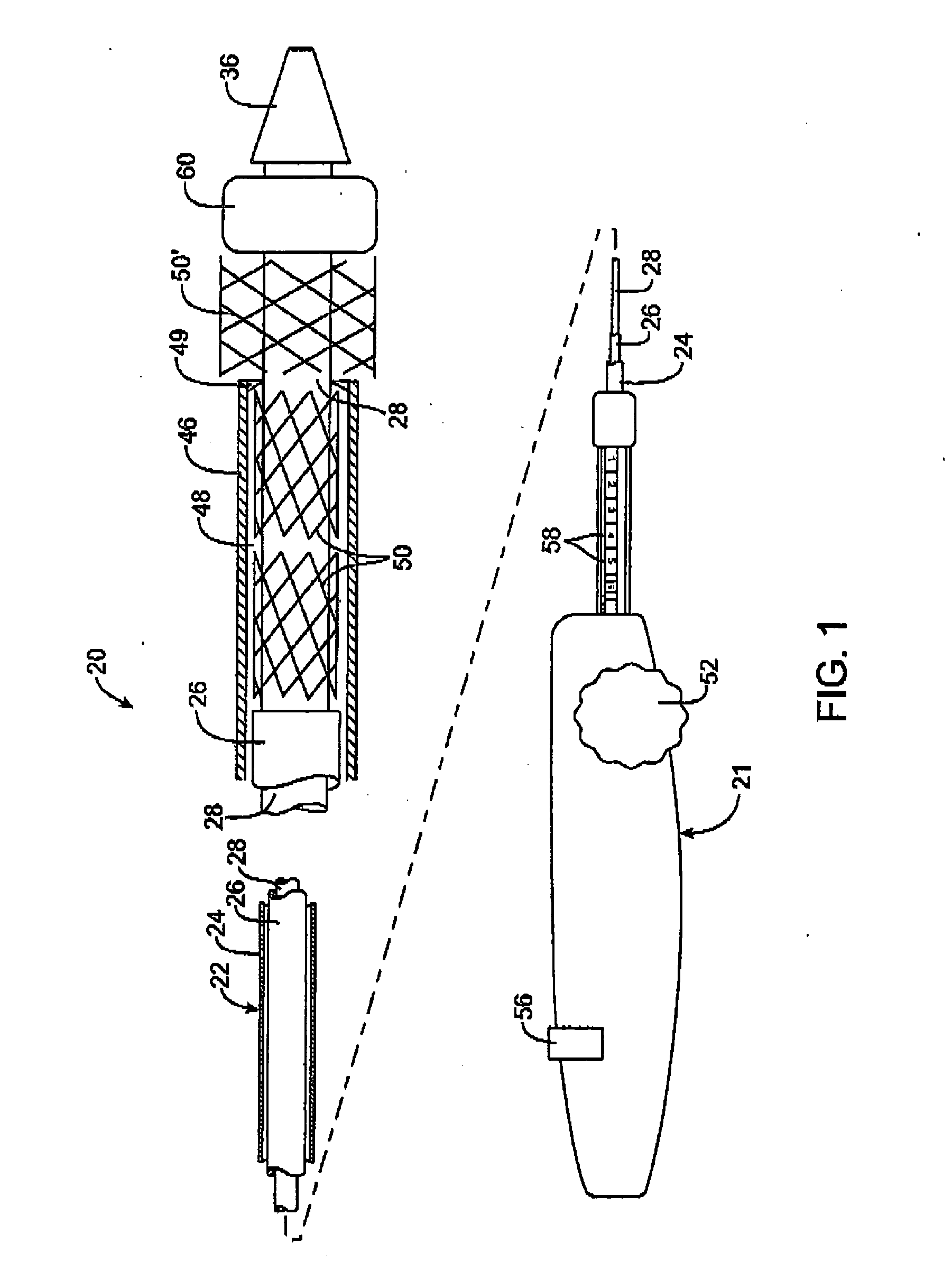
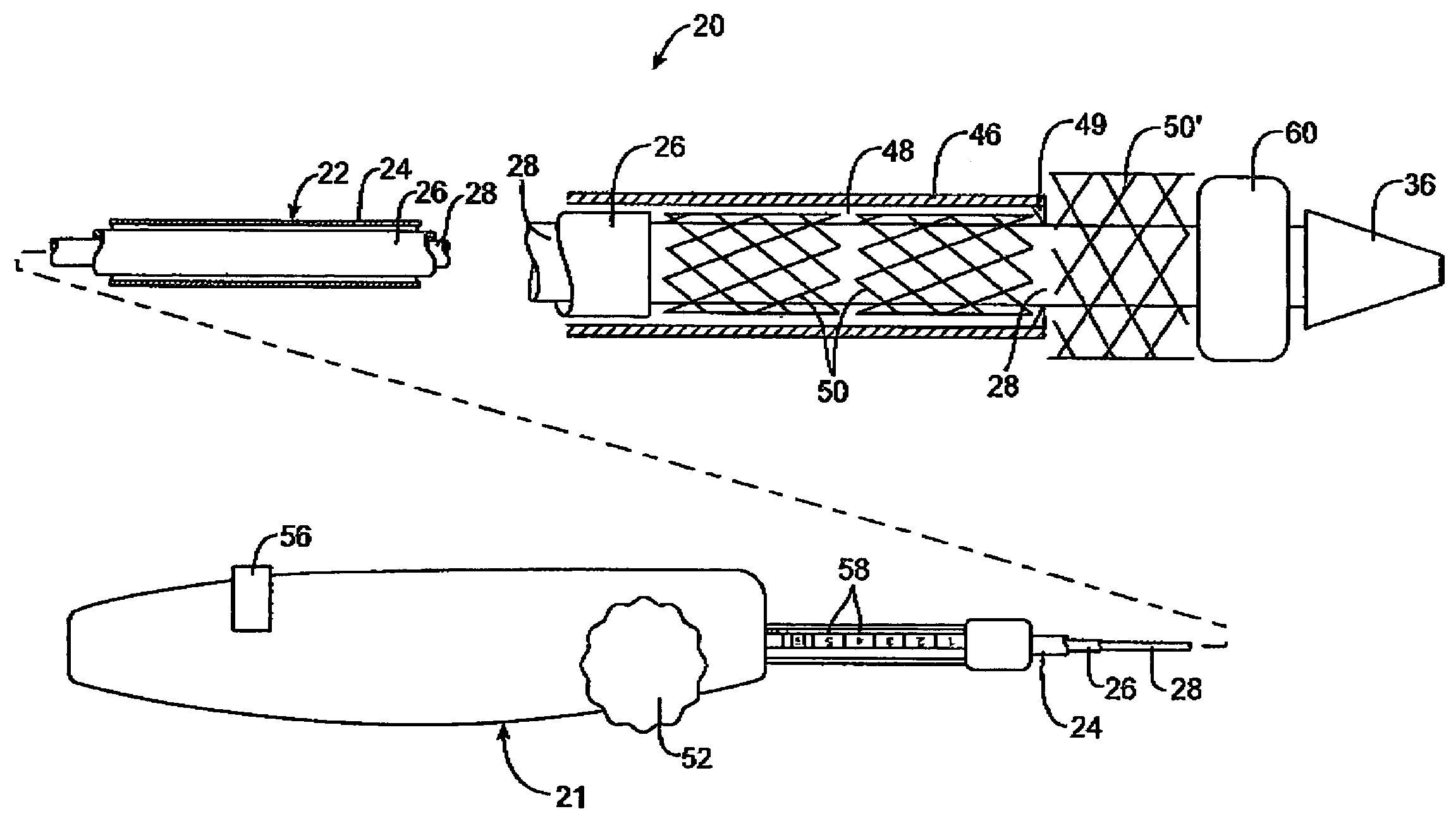
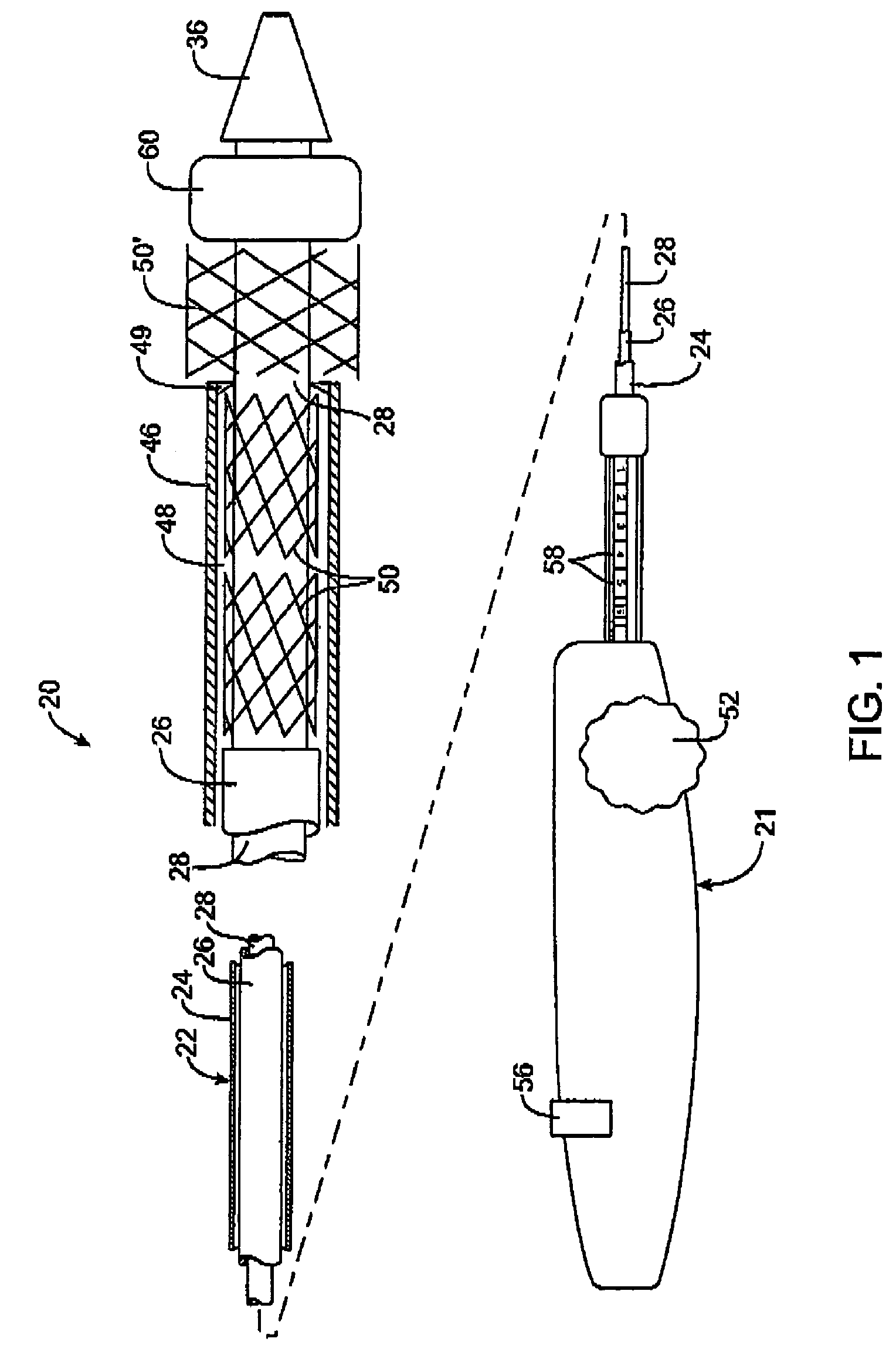
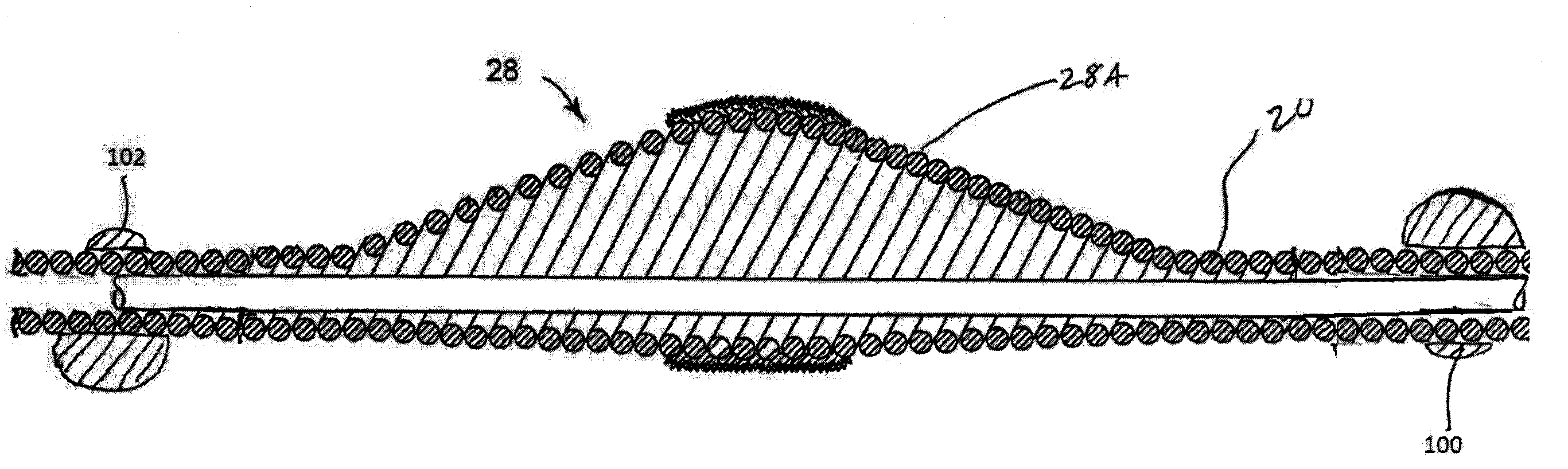
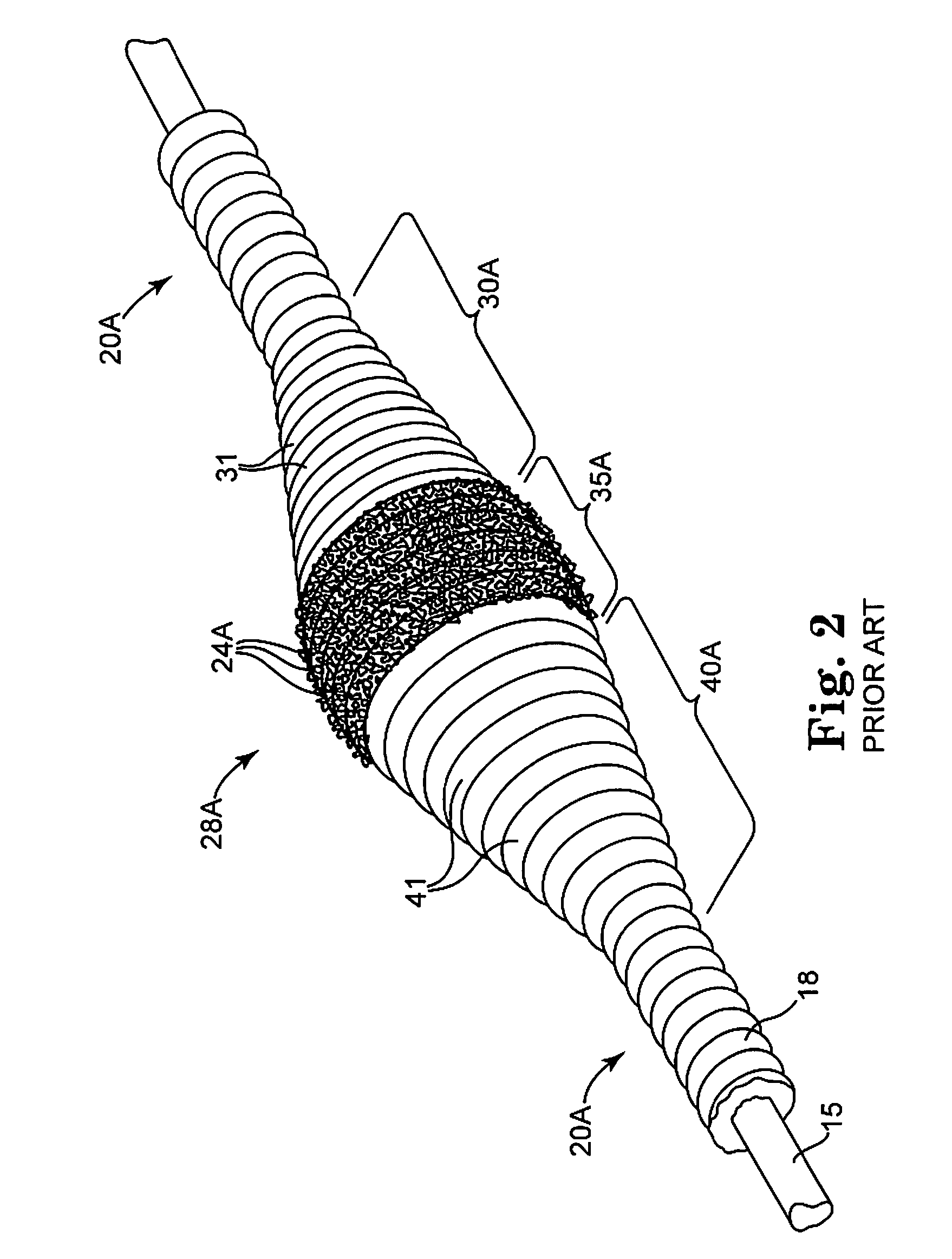
Intravascular deliverable stent for reinforcement of vascular abnormalities
InactiveUS20070168019A1Avoid interactionReduce overall outer diameterStentsCatheterVascular Skin TumorSaphenous veins
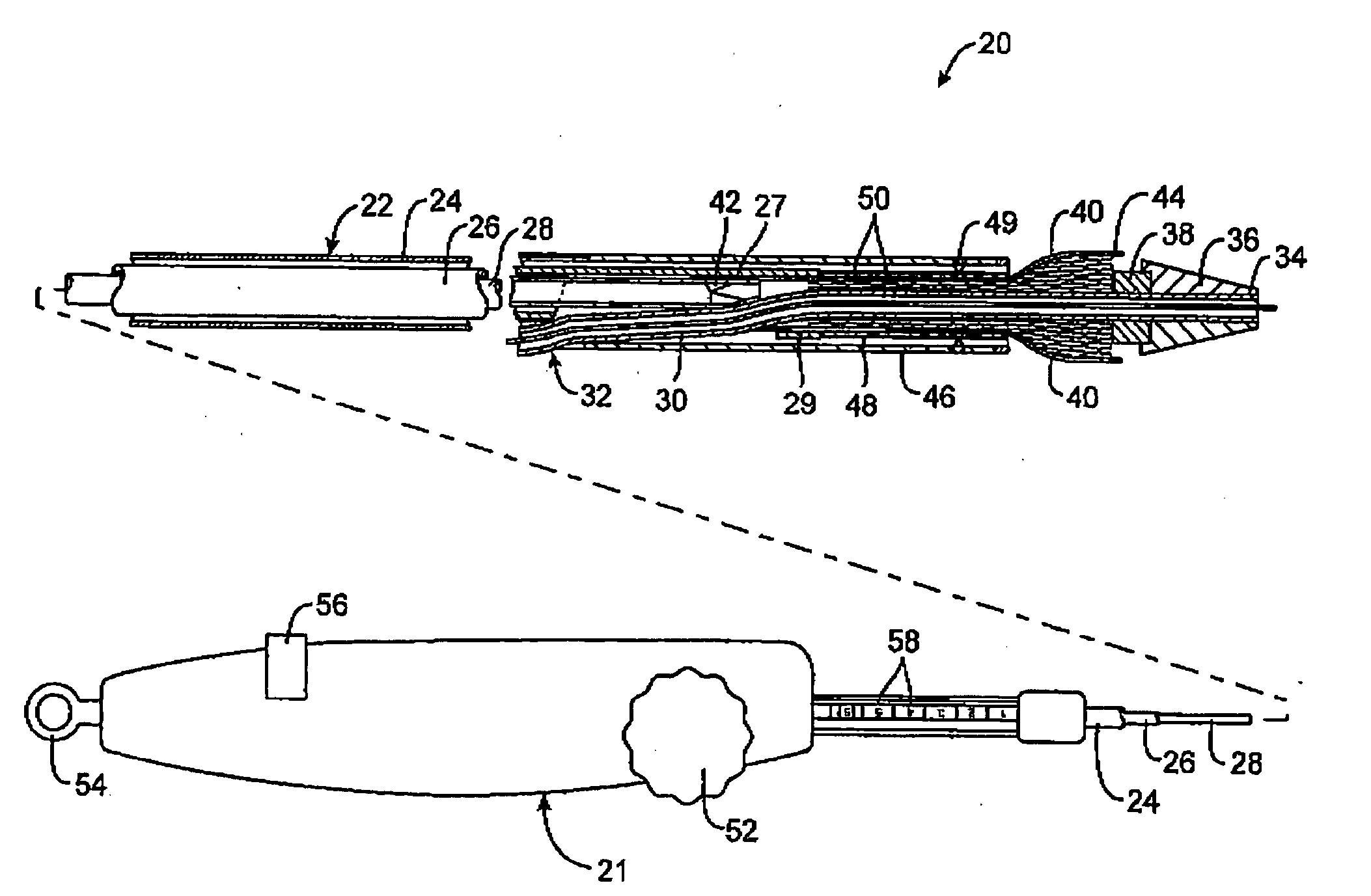
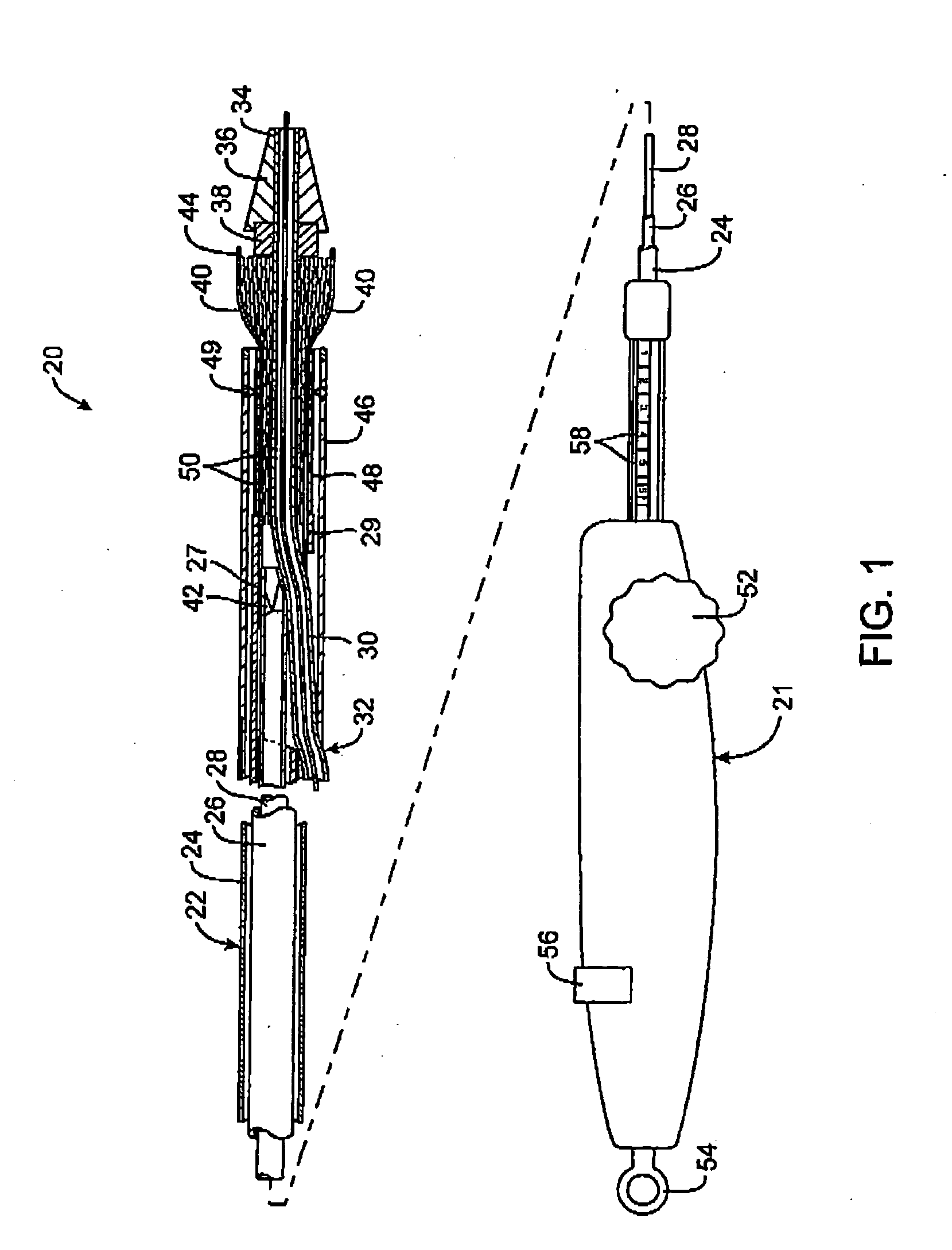
A catheter deliverable stent / graft especially designed to be used in a minimally invasive surgical procedure for treating a variety of vascular conditions such as aneurysms, stenotic lesions and saphenous vein grafts, comprises an innermost tubular structure and at least one further tubular member in coaxial arrangement. In one embodiment, the innermost tubular structure is of a length (L1) and is formed by braiding a relatively few strands of highly elastic metallic alloy. The pick and pitch of the braid are such as to provide relative large fenestrations in the tubular wall that permit blood flow through the wall and provide the primary radial support structure. A portion of the innermost tubular structure of a length L1 is surrounded by a further braided tubular structure having relatively many strands that substantially inhibit blood flow through the fenestrations of the innermost tubular structure. The composite structure can be stretched to reduce the outer diameter of the stent / graft, allowing it to be drawn into a lumen of a delivery catheter. The catheter can then be advanced through the vascular system to the site of treatment and then released, allowing it to self-expand against the vessel wall. Various optional embodiments are disclosed that allow one skilled in the art to tailor the design to the specific application.
Owner:ST JUDE MEDICAL CARDILOGY DIV INC
Devices, systems, and methods for removing targeted lesions from vessels
ActiveUS20100222786A1Easy to operate and controlFacilitate one or more fluid injectionsStentsCannulasStenotic lesionTyping
Devices, systems, and methods for removing targeted lesions from vessels. In at least one embodiment of a device for removing a stenotic lesion from a vessel, the device comprises a sizing portion capable of measuring a luminal size parameter when at least part of the device is positioned within a lumen of a luminal organ, a typing portion, wherein at least part of the at least one typing portion is capable of physically touching a portion of the luminal organ or a structure therein, and a treatment portion capable of removing at least part of a stenotic lesion from the luminal organ.
Owner:3DT HLDG
Custom-length self-expanding stent delivery systems with stent bumpers
ActiveUS20050288763A1Inhibit migrationStable in vesselStentsBlood vesselsStenotic lesionCoronary arteries
Custom-length self-expanding stent delivery systems and methods enable precise control of prosthesis position during deployment. The stent delivery systems carry multiple stent segments and include a stent bumper for helping control the axial position of the stent segments during deployment. This enables the deployment of multiple prostheses at a target site with precision and predictability, preventing stent segment recoil and ejection from the delivery device and thus eliminating excessive spacing or overlap between prostheses. In particular embodiments, the prostheses of the invention are deployed in stenotic lesions in coronary or peripheral arteries or in other vascular locations.
Owner:JW MEDICAL SYSTEMS LTD
Custom-length self-expanding stent delivery systems with stent bumpers
Custom-length self-expanding stent delivery systems and methods enable precise control of prosthesis position during deployment. The stent delivery systems carry multiple stent segments and include a stent bumper for helping control the axial position of the stent segments during deployment. This enables the deployment of multiple prostheses at a target site with precision and predictability, preventing stent segment recoil and ejection from the delivery device and thus eliminating excessive spacing or overlap between prostheses. In particular embodiments, the prostheses of the invention are deployed in stenotic lesions in coronary or peripheral arteries or in other vascular locations.
Owner:JW MEDICAL SYSTEMS LTD
Eccentric abrading head for high-speed rotational atherectomy devices
ActiveUS20080306498A1Improve abilitiesMinimal traumaCannulasExcision instrumentsStenotic lesionRotational axis
The invention provides a rotational atherectomy device having, in various embodiments, a flexible, elongated, rotatable drive shaft with at least one flexible eccentric enlarged abrading head attached thereto. In other embodiments, the eccentric abrading head is not flexible or partially flexible. At least part of the eccentric enlarged cutting head has a tissue removing surface—typically an abrasive surface. In certain embodiments, the abrading head will be at least partially hollow. When placed within an artery against stenotic tissue and rotated at sufficiently high speeds the eccentric nature of the enlarged cutting head causes the cutting head and drive shaft to rotate in such a fashion as to open the stenotic lesion to a diameter substantially larger than the outer diameter of the enlarged cutting head. Preferably the eccentric enlarged cutting head has a center of mass spaced radially from the rotational axis of the drive shaft, facilitating the ability of the device to open the stenotic lesion to a diameter substantially larger than the outer diameter of the enlarged cutting head when operated at high speeds.
Owner:CARDIOVASCULAR SYST INC
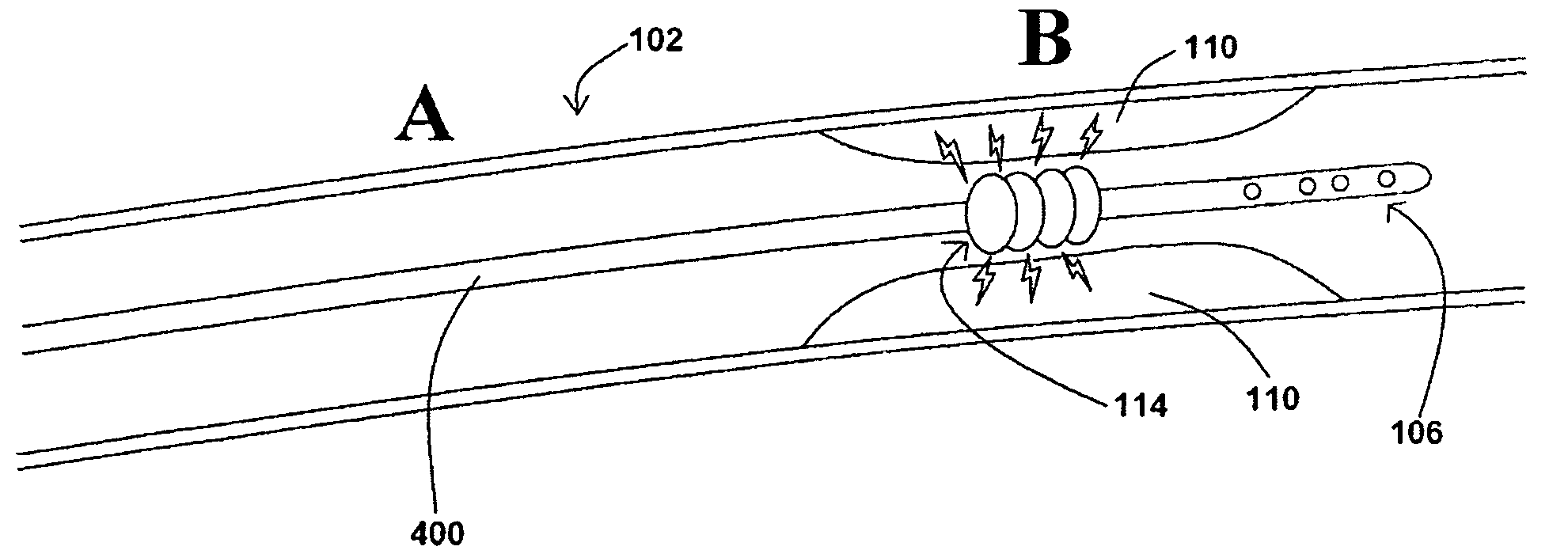
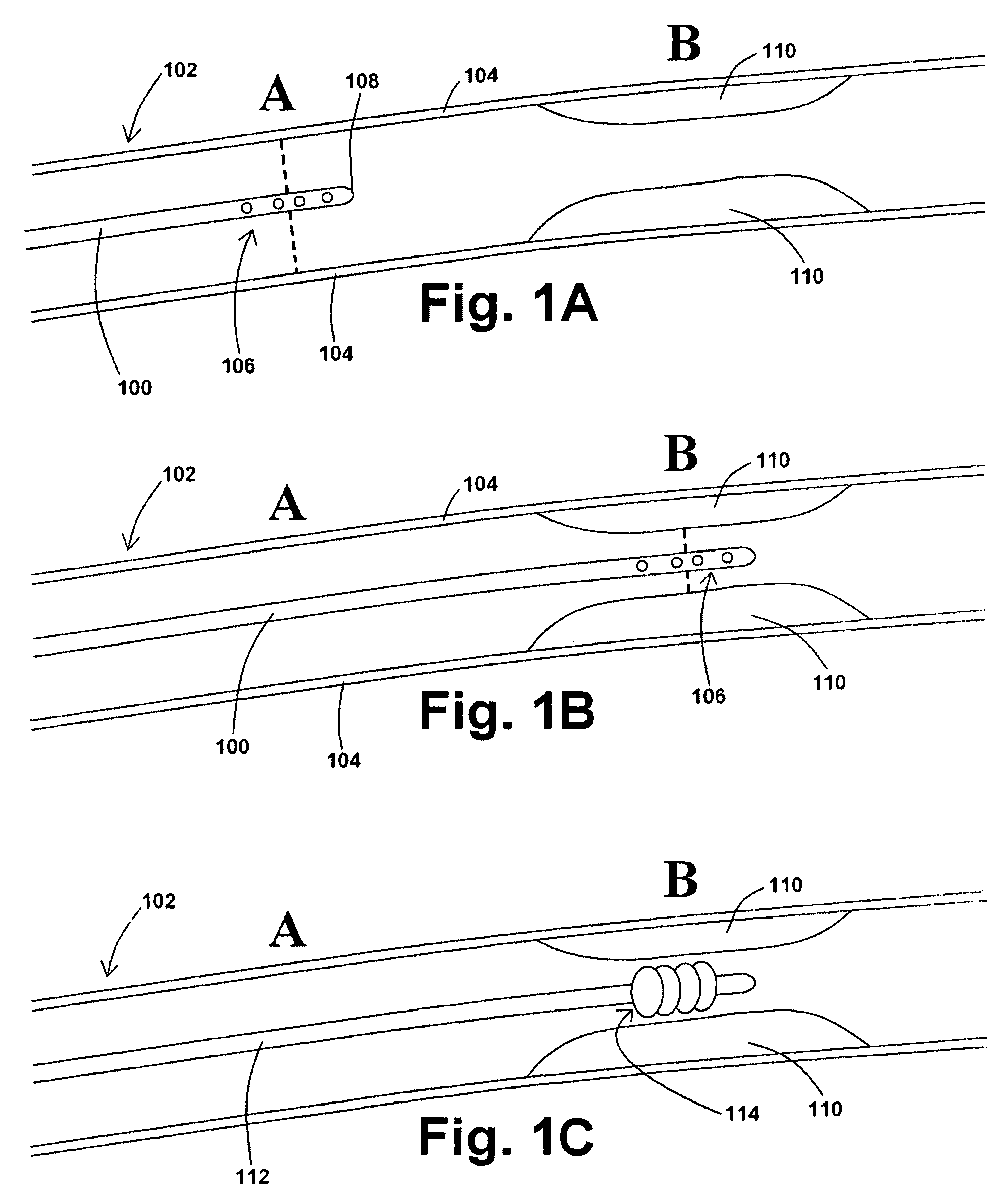
Devices, systems, and methods for removing stenotic lesions from vessels
The present application provides various devices, systems, and methods for removing stenotic lesions from vessels. In at least one embodiment of an exemplary device, the device comprises at least one sizing portion and at least one treatment portion. Such a device may be useful to, for example, remove a stenotic lesion from a vessel by positioning the device within a vessel lumen, operating the sizing portion to obtain luminal size parameter data, operating the treatment portion at a location within the vessel lumen at or near a stenotic lesion, and ceasing operation of the treatment portion when the luminal size parameter data indicates a preferred luminal size parameter.
Owner:3DT HLDG
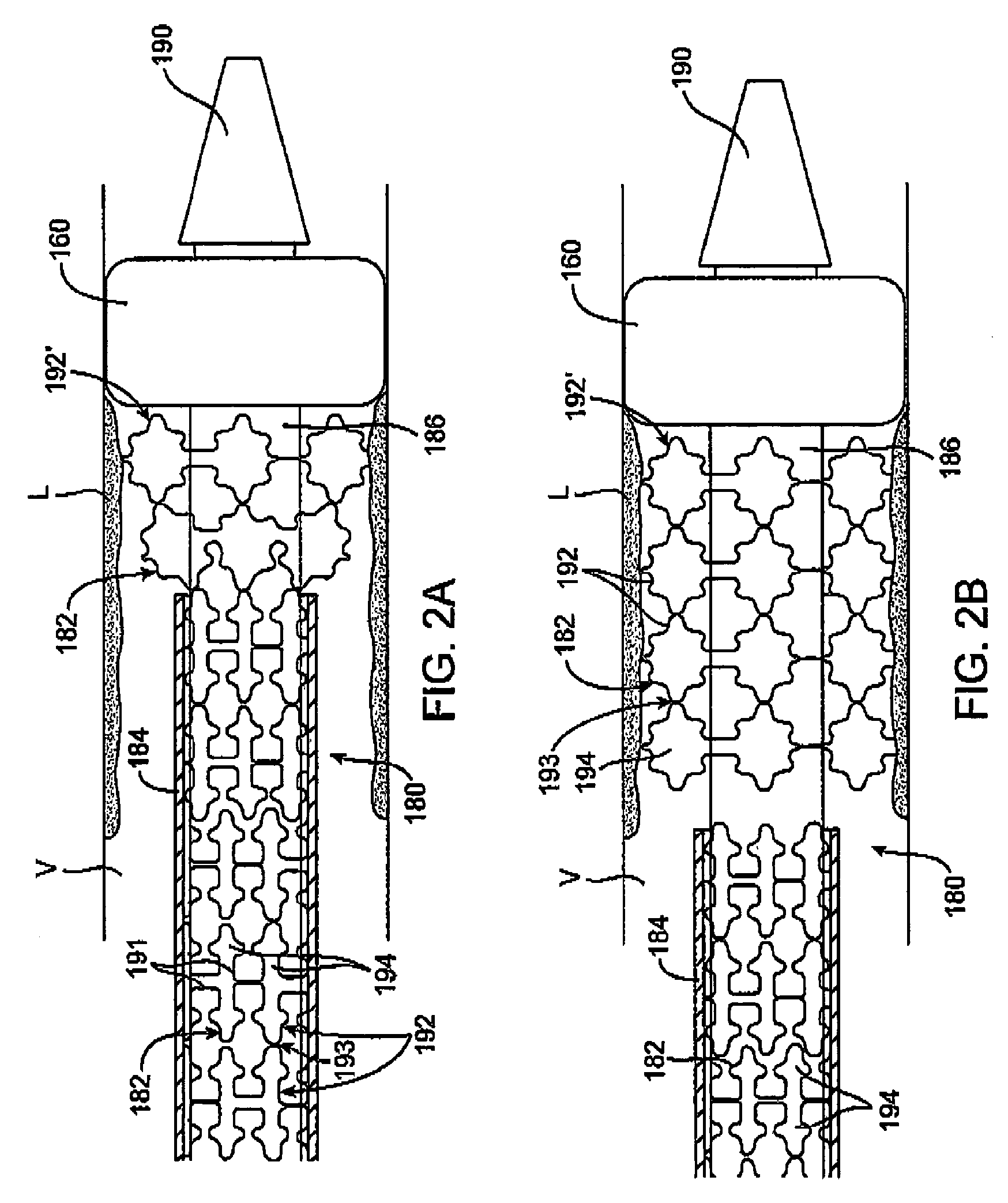
Embolic protection device having a reticulated body with staggered struts
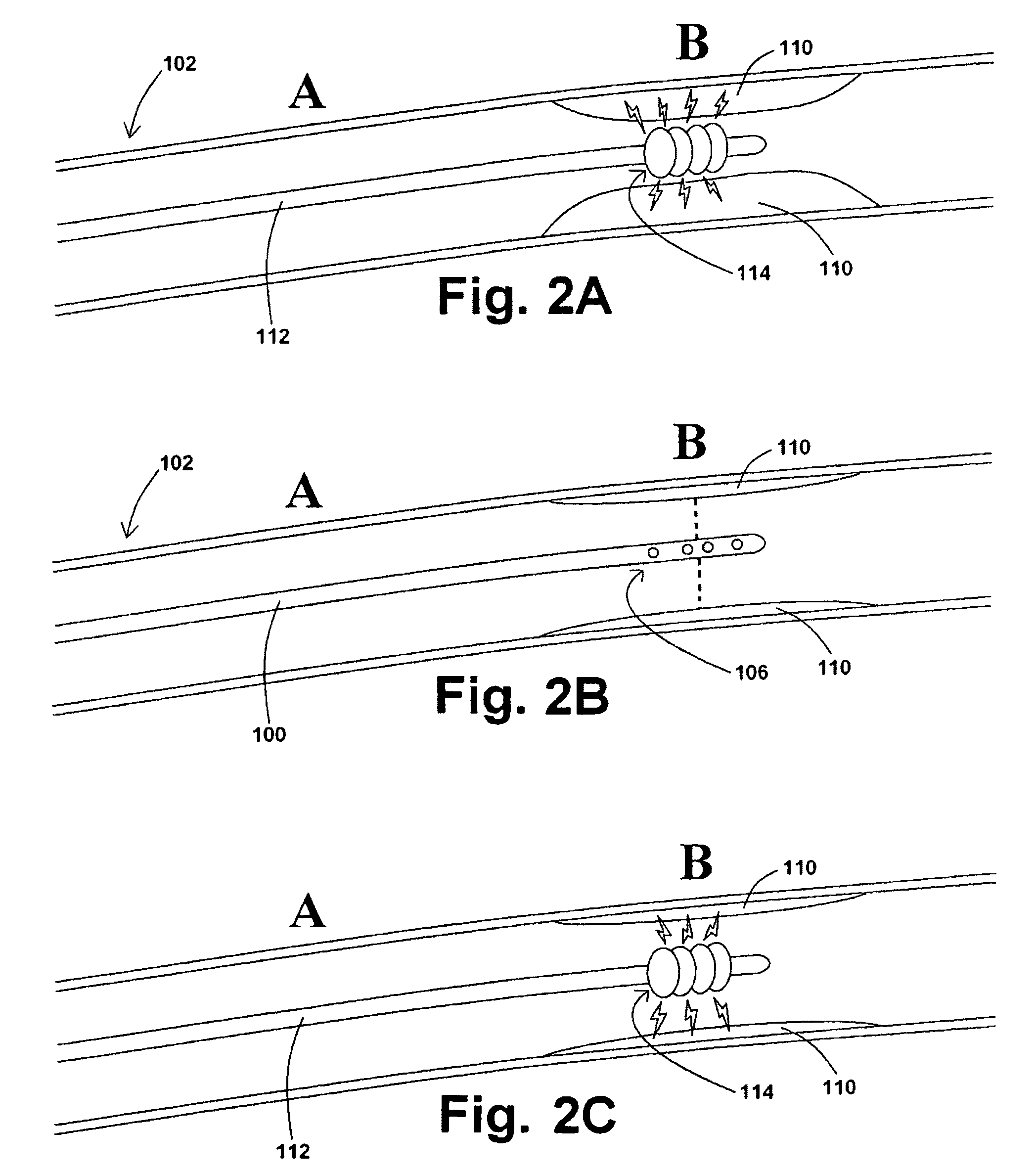
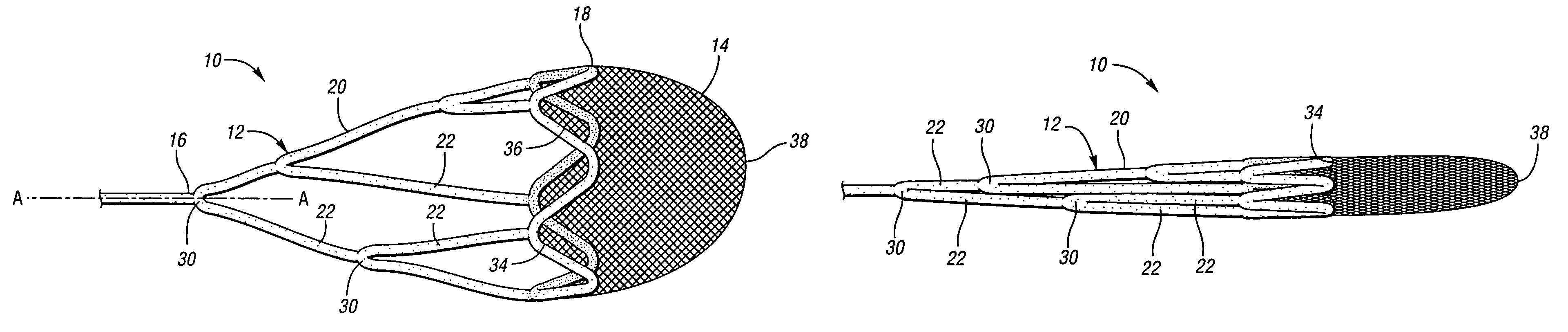
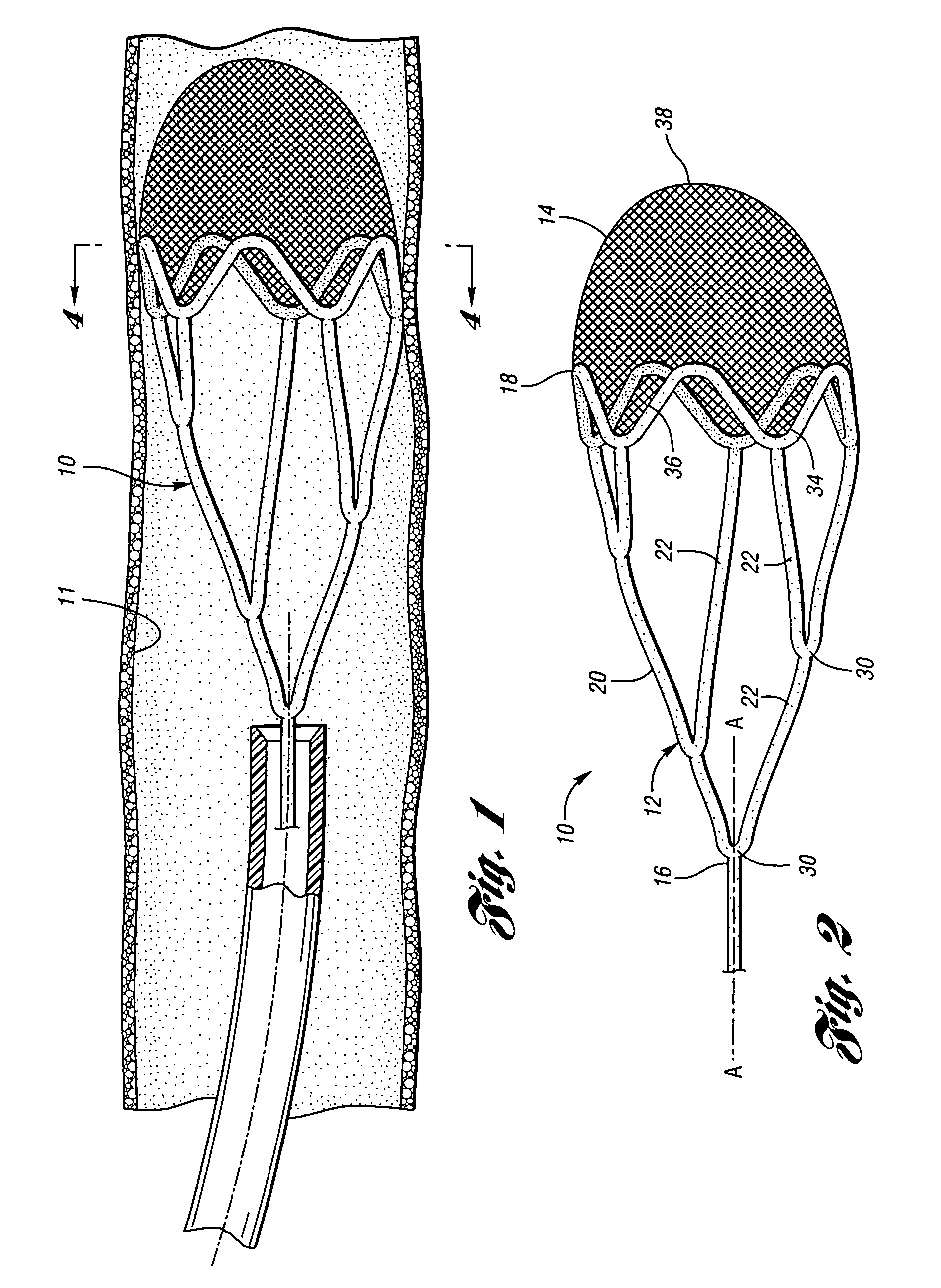
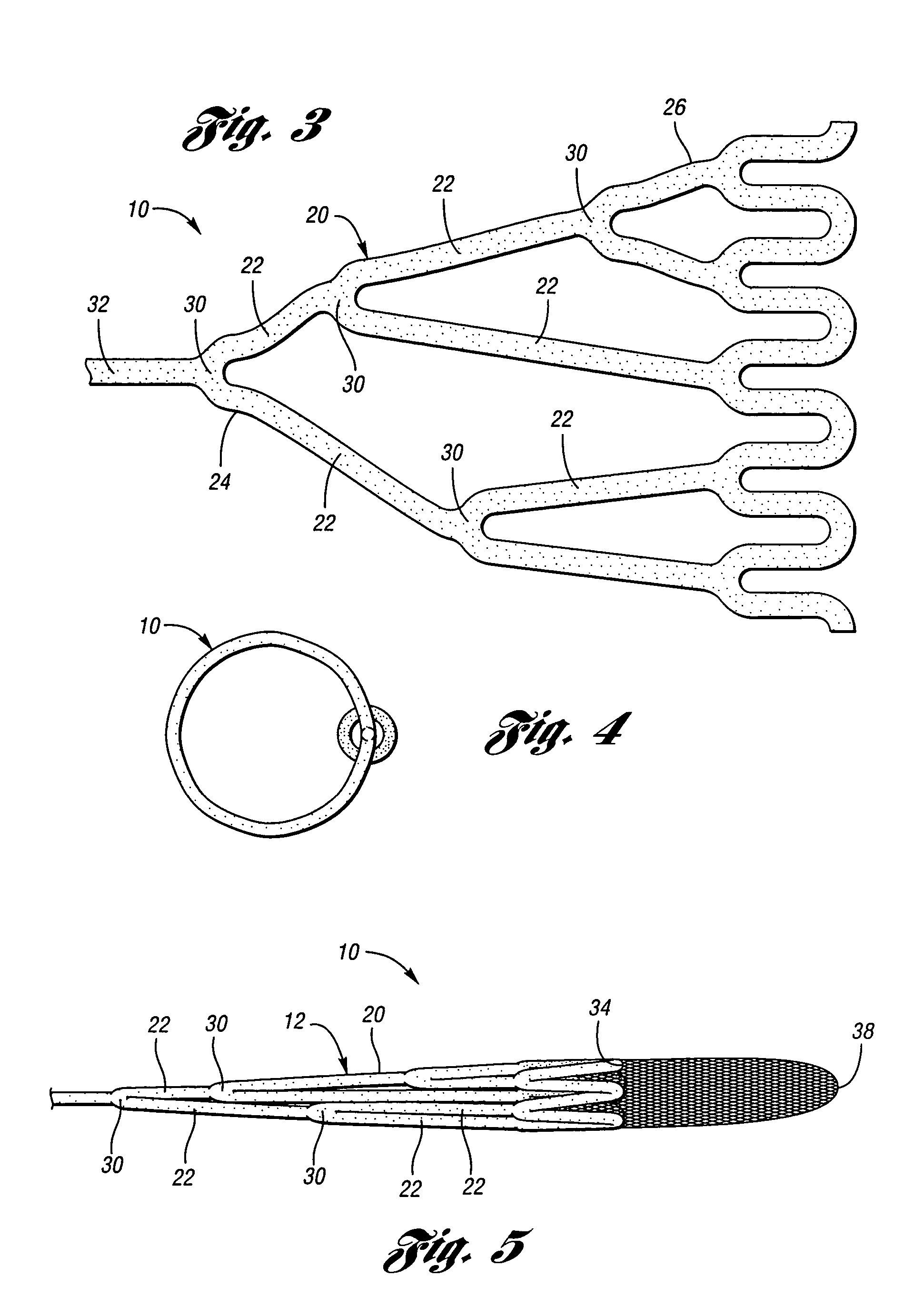
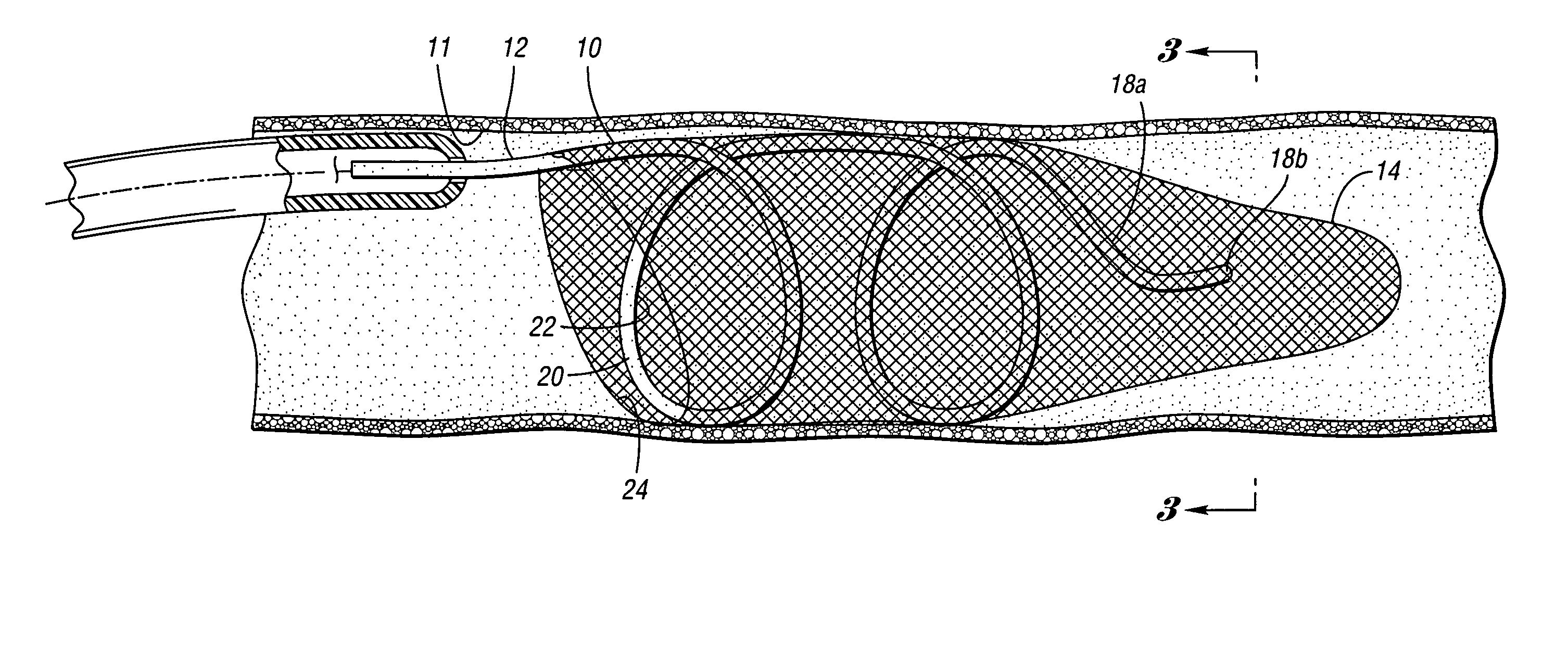
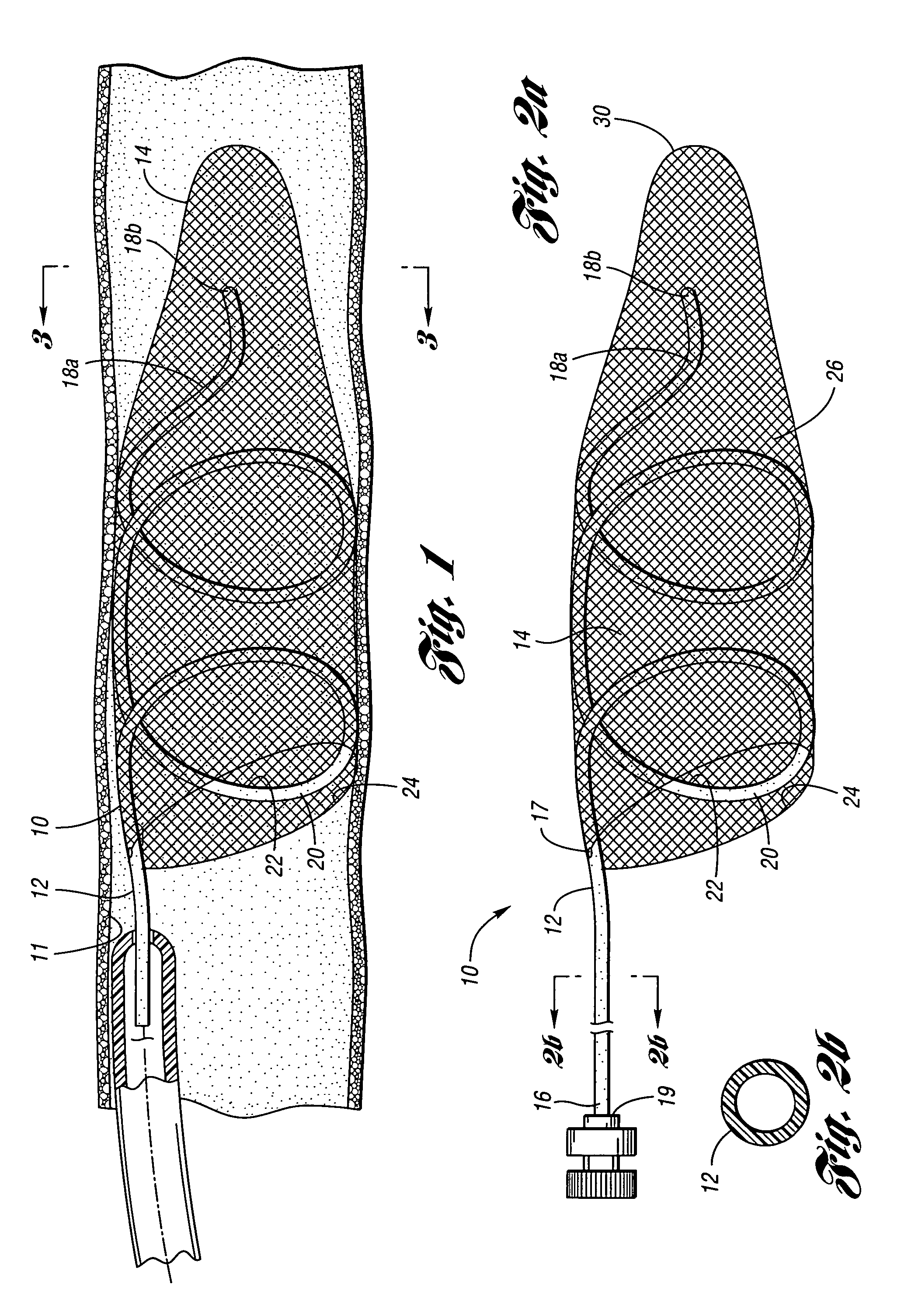
ActiveUS7850708B2Minimizes restricted flowEasy retrievalSurgeryDilatorsStenotic lesionEmbolic Protection Devices
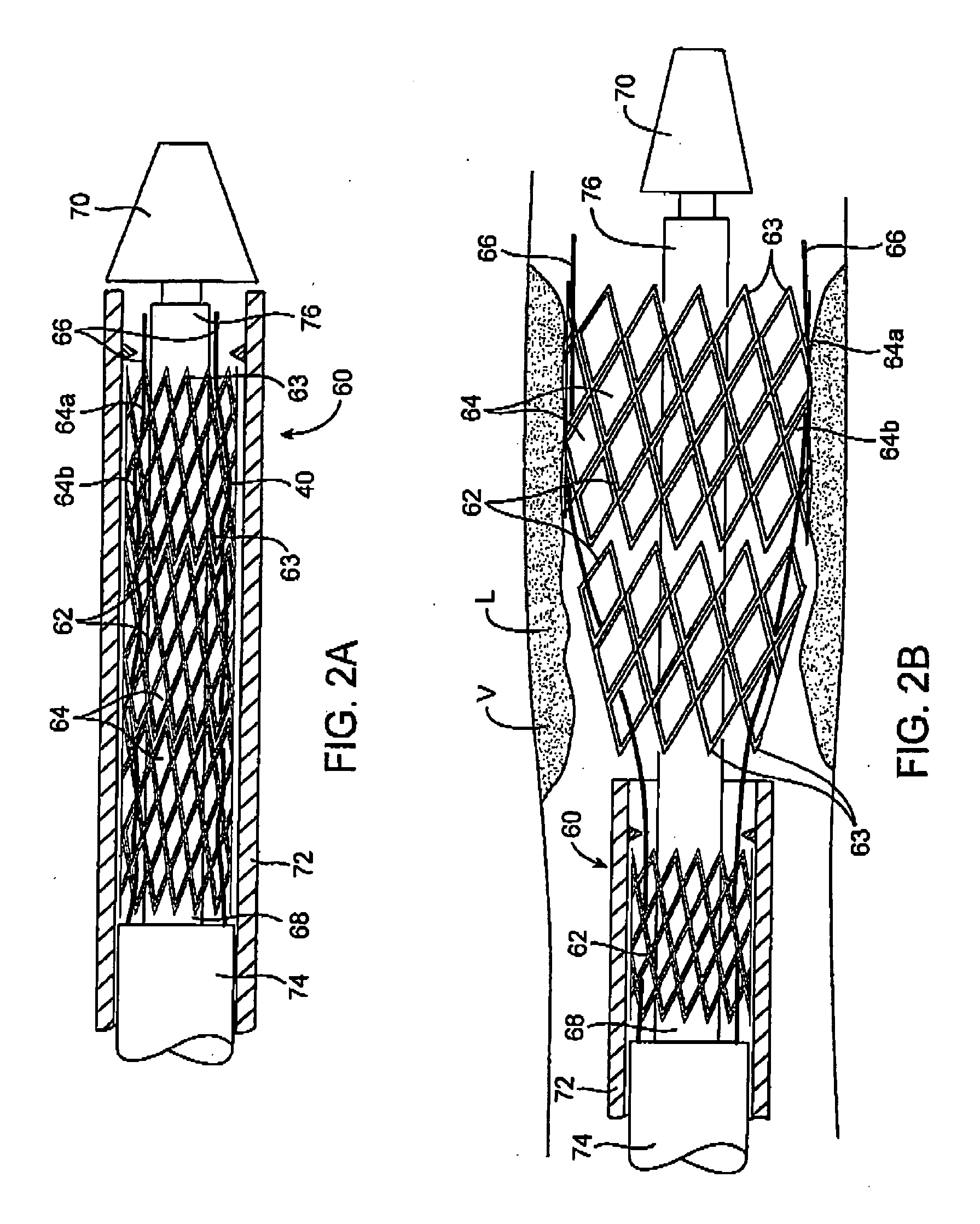
An embolic protection device for capturing emboli during treatment of a stenotic lesion in a body vessel is disclosed. The device comprises a basket and a filter attached to the basket. The basket has a deployed state and an undeployed state. The basket includes a reticulated body having an outer diameter. The reticulated body includes a plurality of struts connected together in a singly staggered configuration distally along a longitudinal axis to a distal end. The plurality of struts of the reticulated body is configured to fold along the longitudinal axis. The basket has a proximal stem proximally extending from the body. The filter portion has a lip attached to the distal end defining an opening of the filter portion when the basket is in the deployed state for capturing emboli. The filter portion extends from the lip to a filter end.
Owner:COOK MEDICAL TECH LLC
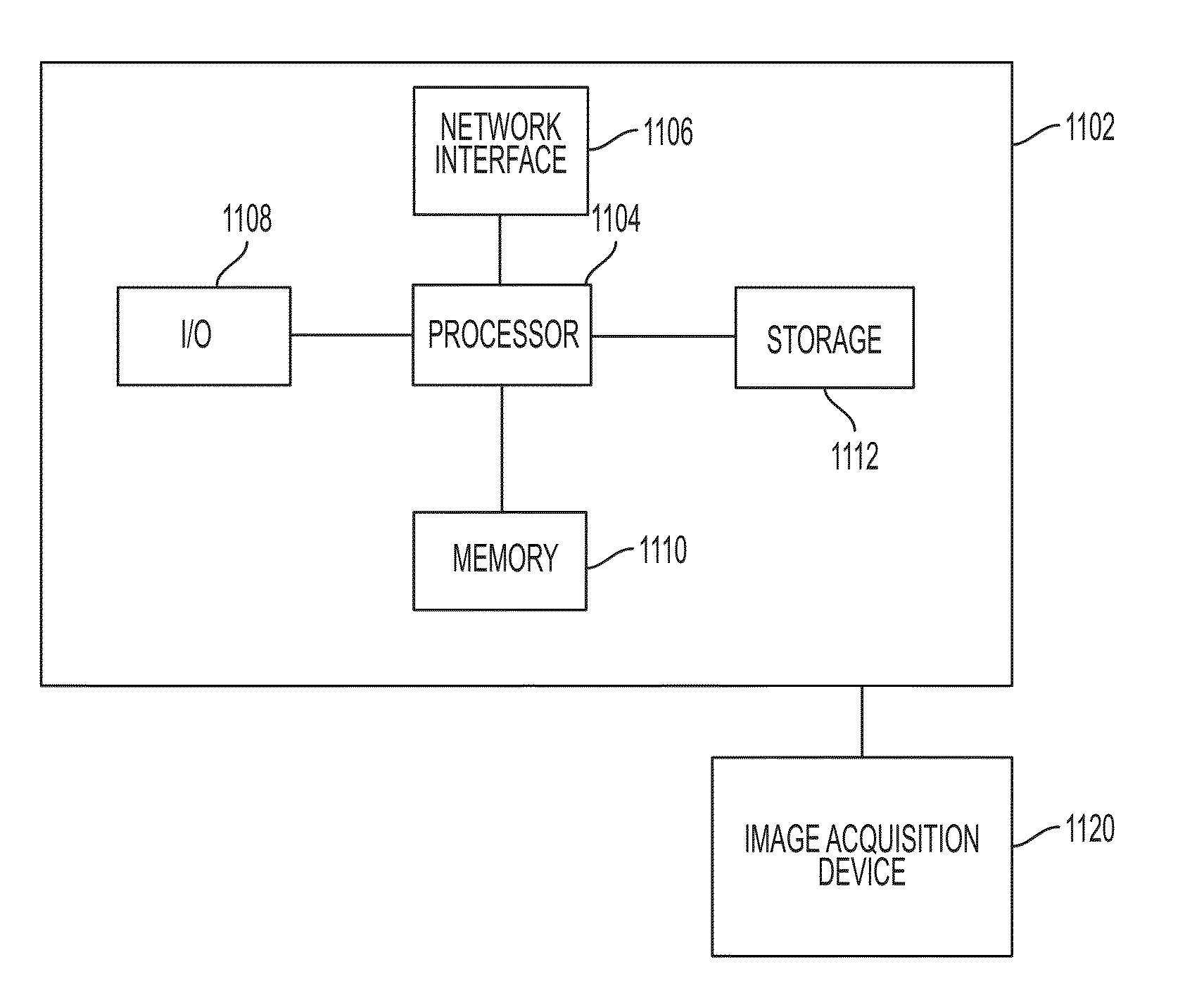
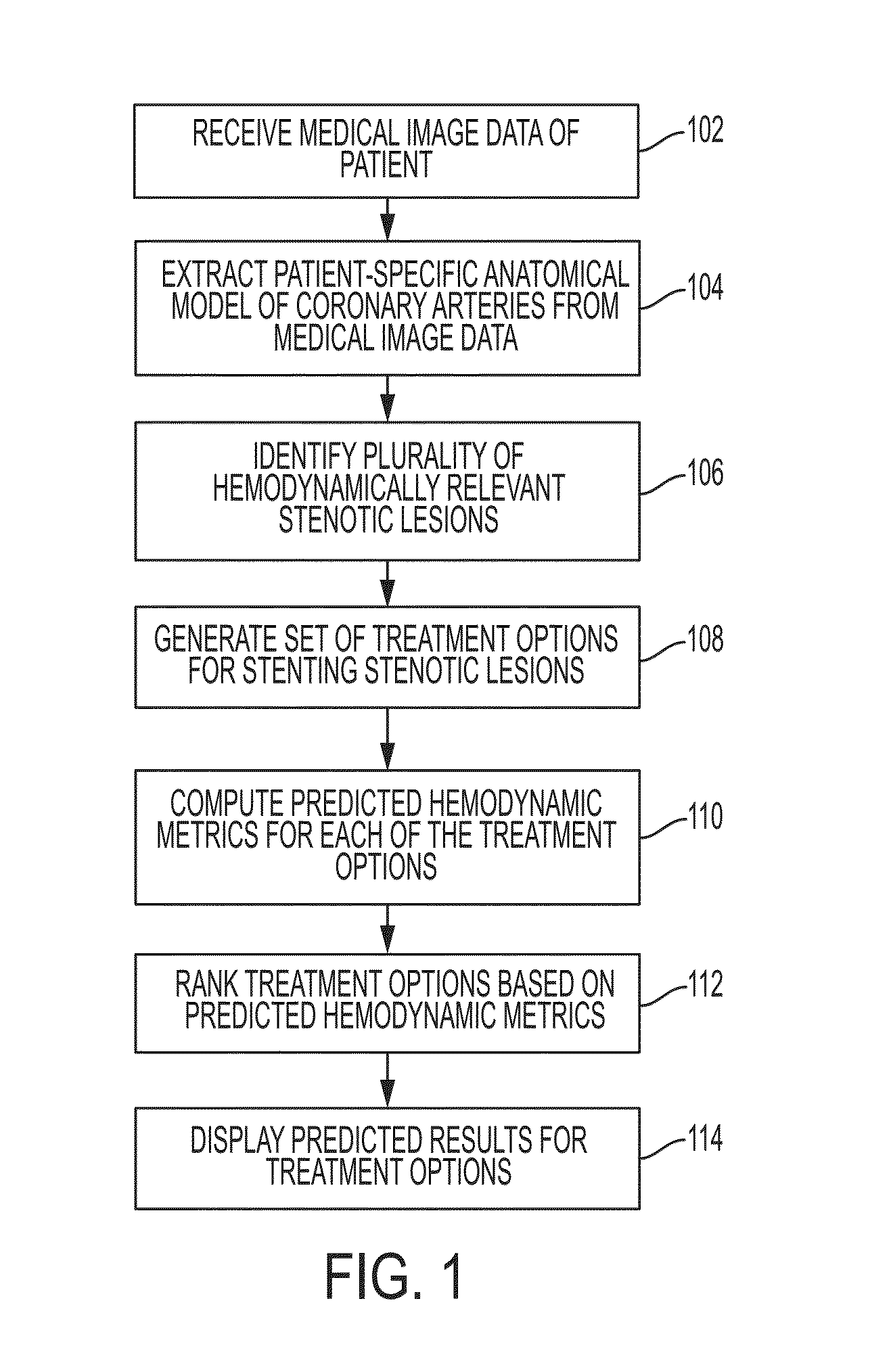
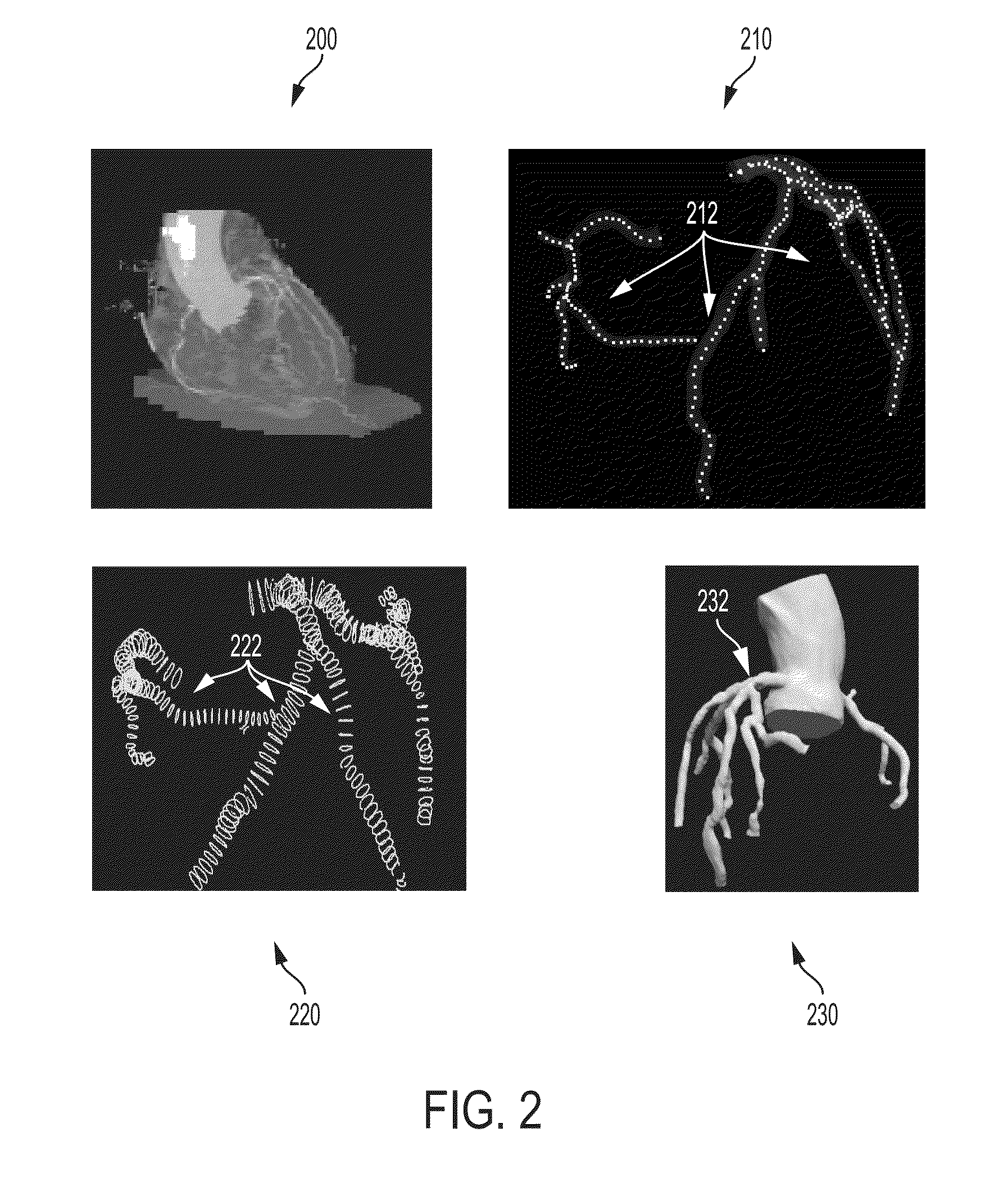
Method and System for Automated Therapy Planning for Arterial Stenosis
A method and system for automated decision support for treatment planning of arterial stenoses is disclosed. A set of stenotic lesions is identified in a patient's coronary arteries from medical image data of the patient. A plurality of treatment options are generated for the set of stenotic lesions, wherein each of the plurality of treatment options corresponds to a stenting configuration in which one or more of the stenotic lesions are stented. For each of the plurality of treatment options, predicted hemodynamic metrics for the set of stenotic lesions resulting from the stenting configuration corresponding to that treatment option are calculated.
Owner:SIEMENS HEALTHCARE GMBH
Rotational atherectomy device and method to improve abrading efficiency
ActiveUS8632557B2Good curative effectConvenient restCannulasExcision instrumentsRotational axisStenotic lesion
Owner:CARDIOVASCULAR SYST INC
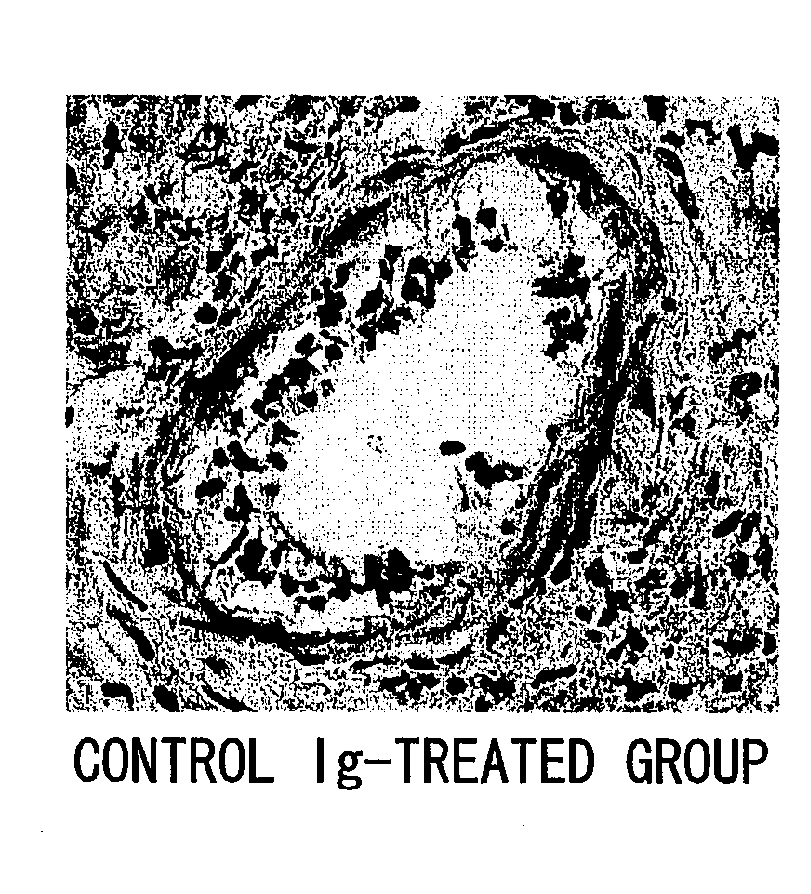
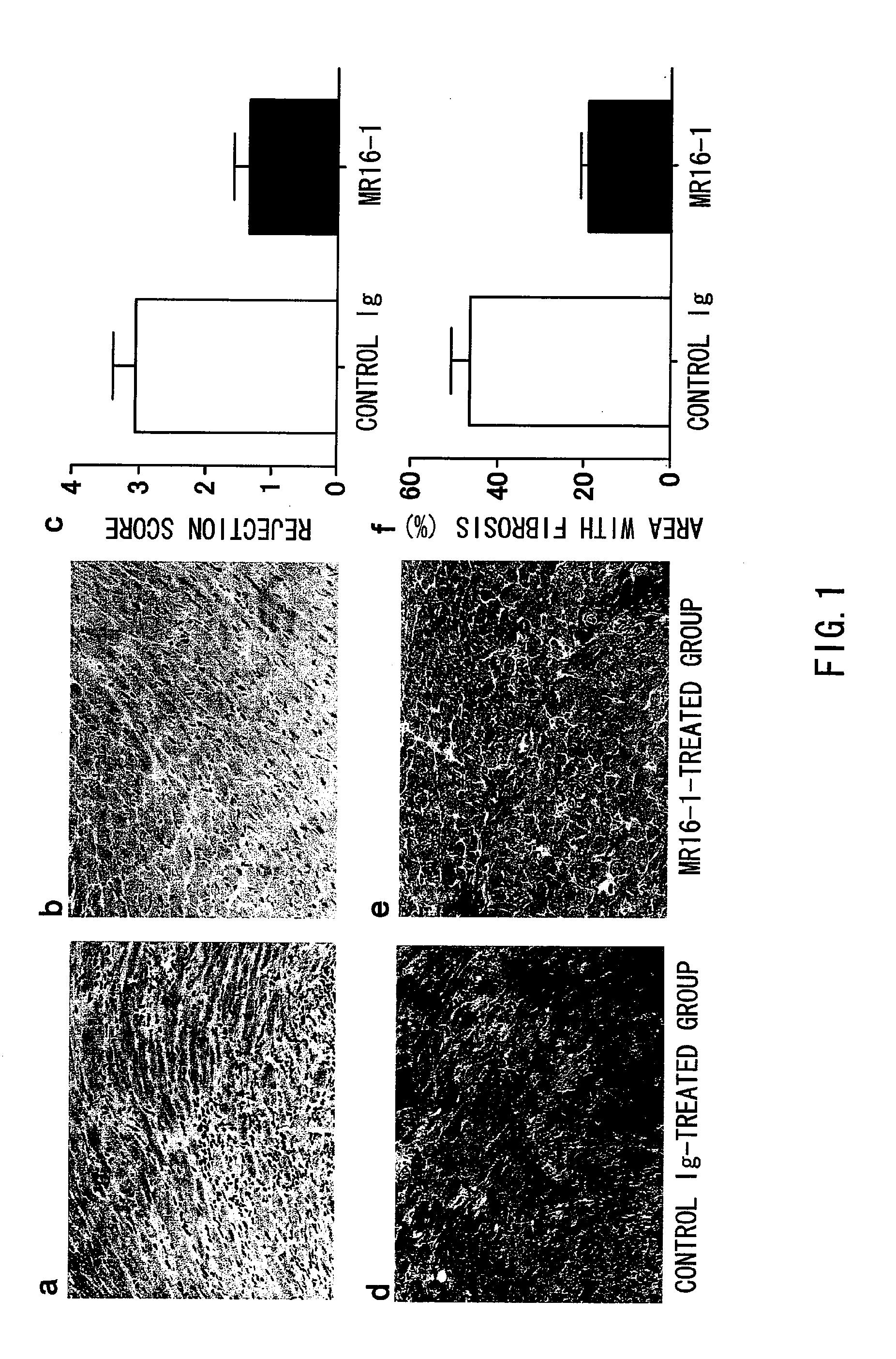
Chronic Rejection Inhibitor
ActiveUS20100061986A1Suppressing chronic rejection reactionAvoid spreadingAntibody ingredientsImmunoglobulinsHeart transplantationStenotic lesion
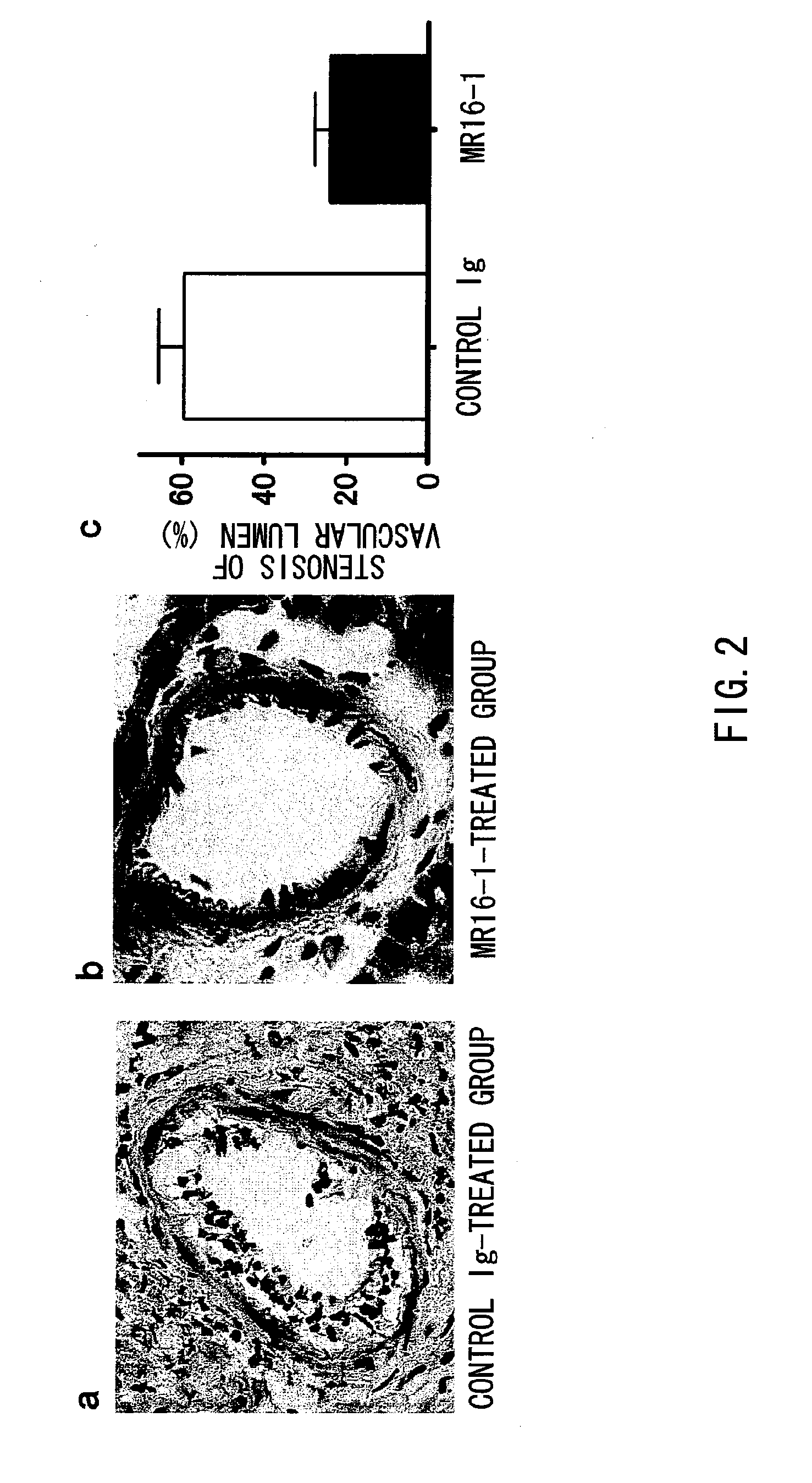
The present inventors assessed the effect of anti-IL-6 receptor antibodies in suppressing chronic rejection reaction. They assessed the effect of anti-mouse IL-6 receptor antibody (MR16-1) administration in suppressing the chronic rejection reaction using a mouse model for post-heart-transplantation chronic rejection. The result of histopathological analysis of transplanted hearts extirpated 60 days after transplantation revealed that fibrosis of myocardium and vascular stenotic lesions, which are pathological conditions characteristic of the chronic rejection reaction, were significantly suppressed in the MR16-1-treated group as compared to the control group. Thus, MR16-1 administration was demonstrated to have the effect of suppressing chronic rejection reaction. Specifically, the present inventors discovered for the first time that the rejection reaction in the chronic phase after organ transplantation was suppressed by administering an anti-IL-6 receptor antibody.
Owner:CHUGAI PHARMA CO LTD +1
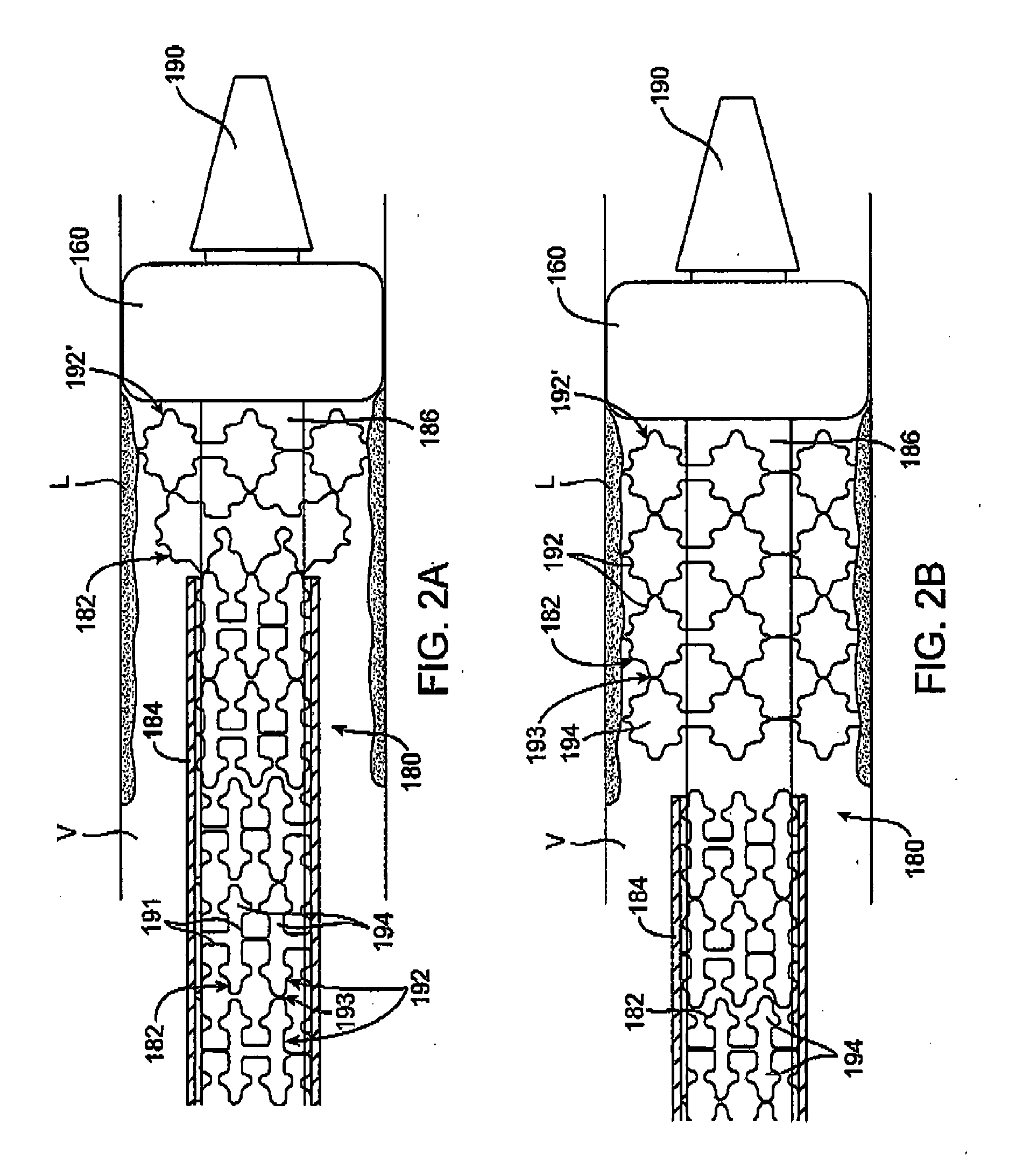
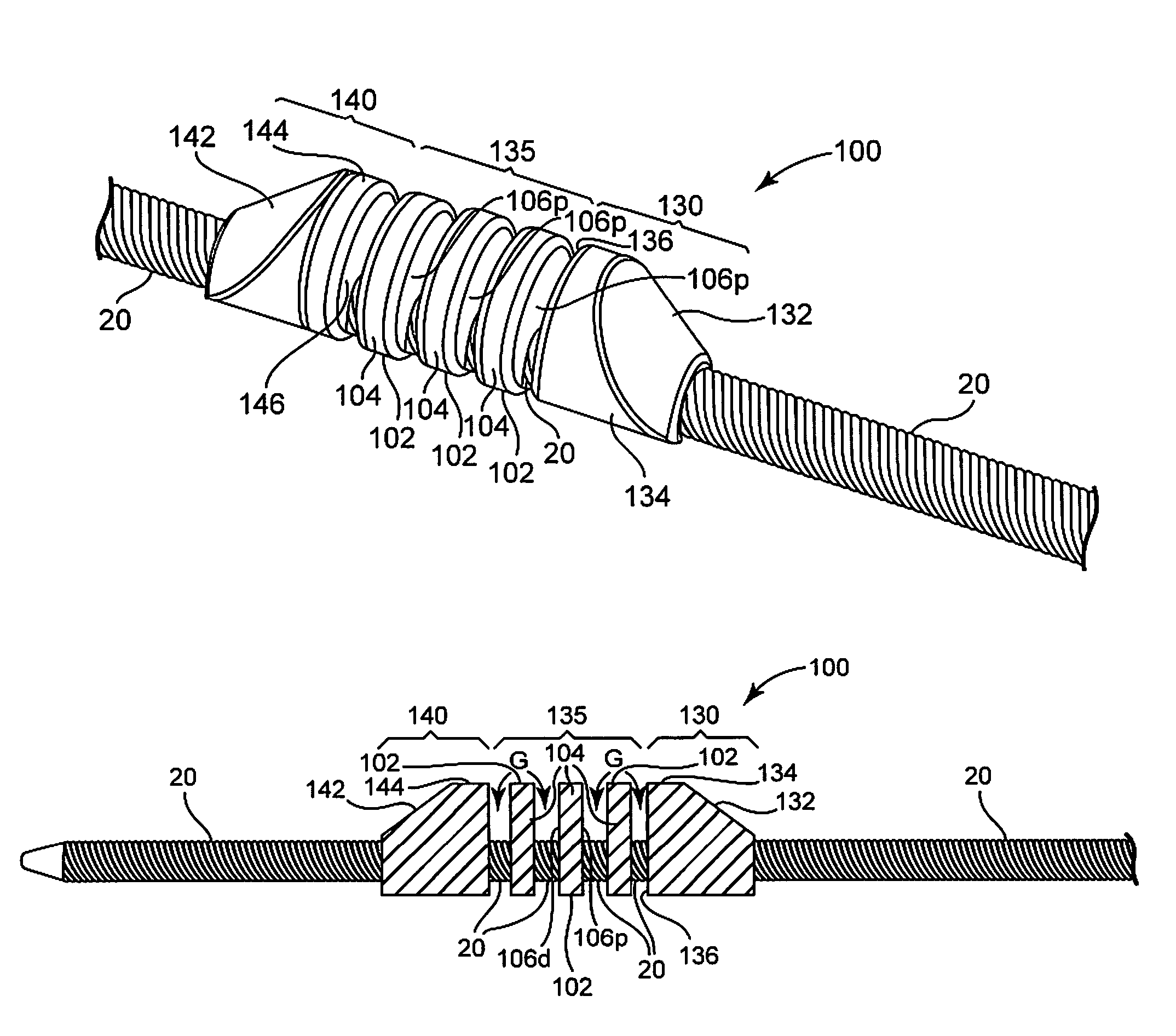
Expandable device for treatment of a stricture in a body vessel
ActiveUS9138307B2Reduce cross-sectional profileIncrease flexibilityGuide needlesExcision instrumentsStenotic lesionSurgery
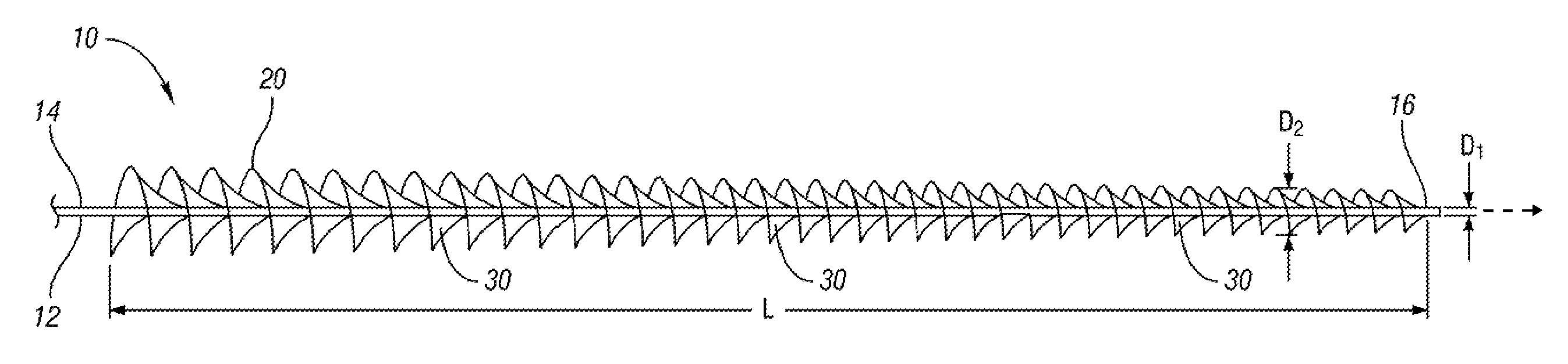
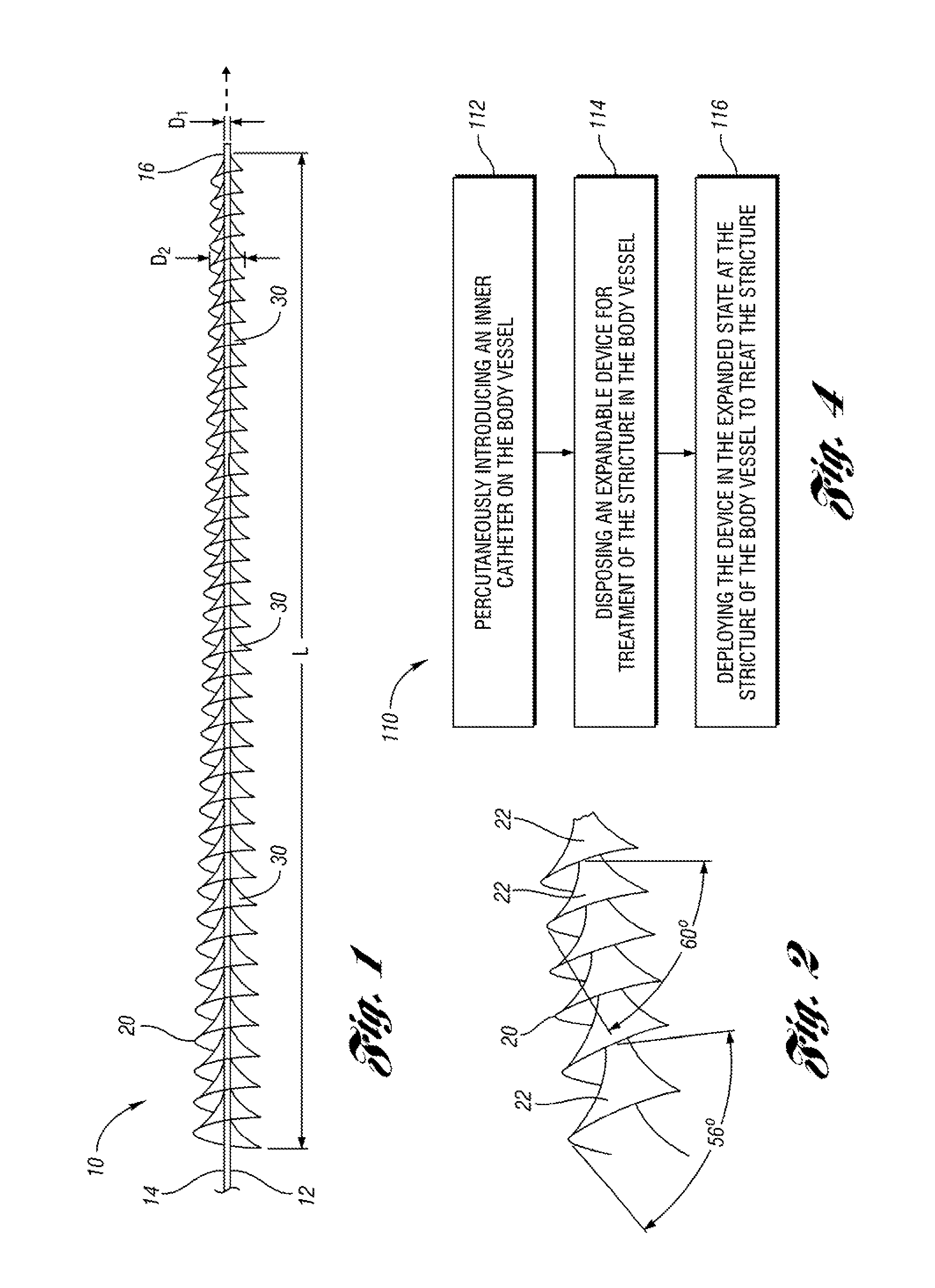
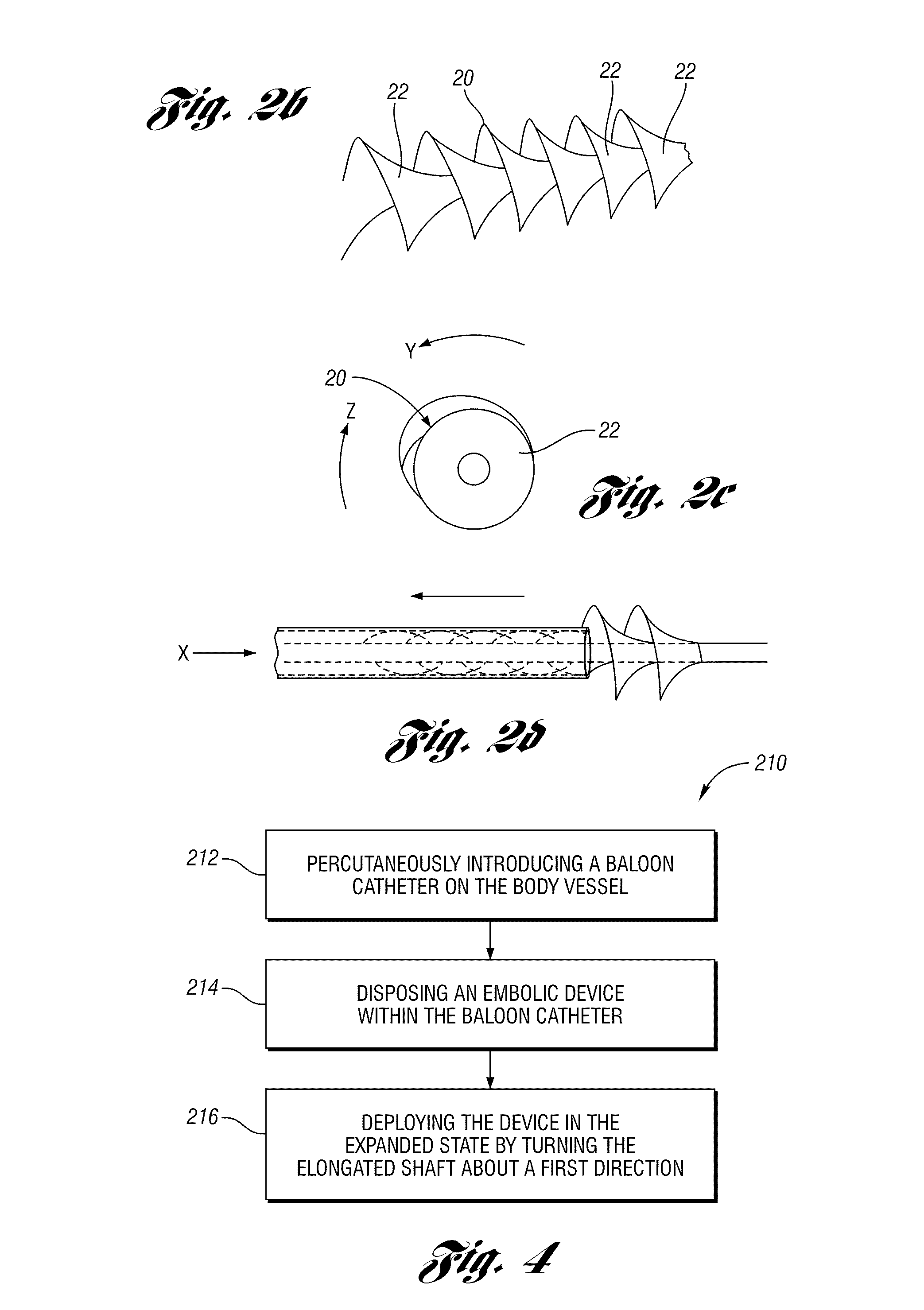
An expandable and retrievable device for treatment of a stenotic lesion in a body vessel is disclosed. The device comprises a tubular portion including a proximal end and a distal end extending from the proximal end. The tubular portion has a lumen formed therethrough between the proximal and distal ends. The device further comprises an expandable member formed helically about the tubular portion. The expandable member is configured to helically close, defining a collapsed state for delivery of the device. The expandable member is configured to helically open, defining an expanded state for treatment of the stenotic lesion in the body vessel. The expandable member has at least one filter portion that helically extends from the tubular portion at a predetermined angle. This defines a proximally faced opening when the expandable member is in the expanded state.
Owner:COOK MEDICAL TECH LLC
Rotational atherectomy segmented abrading head and method to improve abrading efficiency
ActiveUS20100211088A1Improve abilitiesConvenient restCannulasExcision instrumentsRotational axisStenotic lesion
The invention provides a rotational atherectomy system, device and method having, in various embodiments, a flexible, elongated, rotatable drive shaft comprising an eccentric abrading head comprising at least one eccentric abrading cylindrical segments attached to the drive shaft and in spaced proximity with proximal and a distal conical segments. Each individual abrading segment, comprises a first tissue removing surface, typically an abrasive coating on the outer surface, that is designed to abrade calcified, hard tissue and abrasive coating on the leading and trailing surfaces designed to abrade non-calcified, soft tissue. Each abrading segment, as well as the abrading head comprising the collective segments, has a center of mass spaced radially from the rotational axis of the drive shaft, facilitating the ability of the device to open the stenotic lesion to a diameter larger than the outer diameter of the enlarged abrading head when operated at high speeds.
Owner:CARDIOVASCULAR SYST INC
Helical embolic protection device
ActiveUS8252018B2Small profileEffective maintenanceDilatorsExcision instrumentsStenotic lesionDistal portion
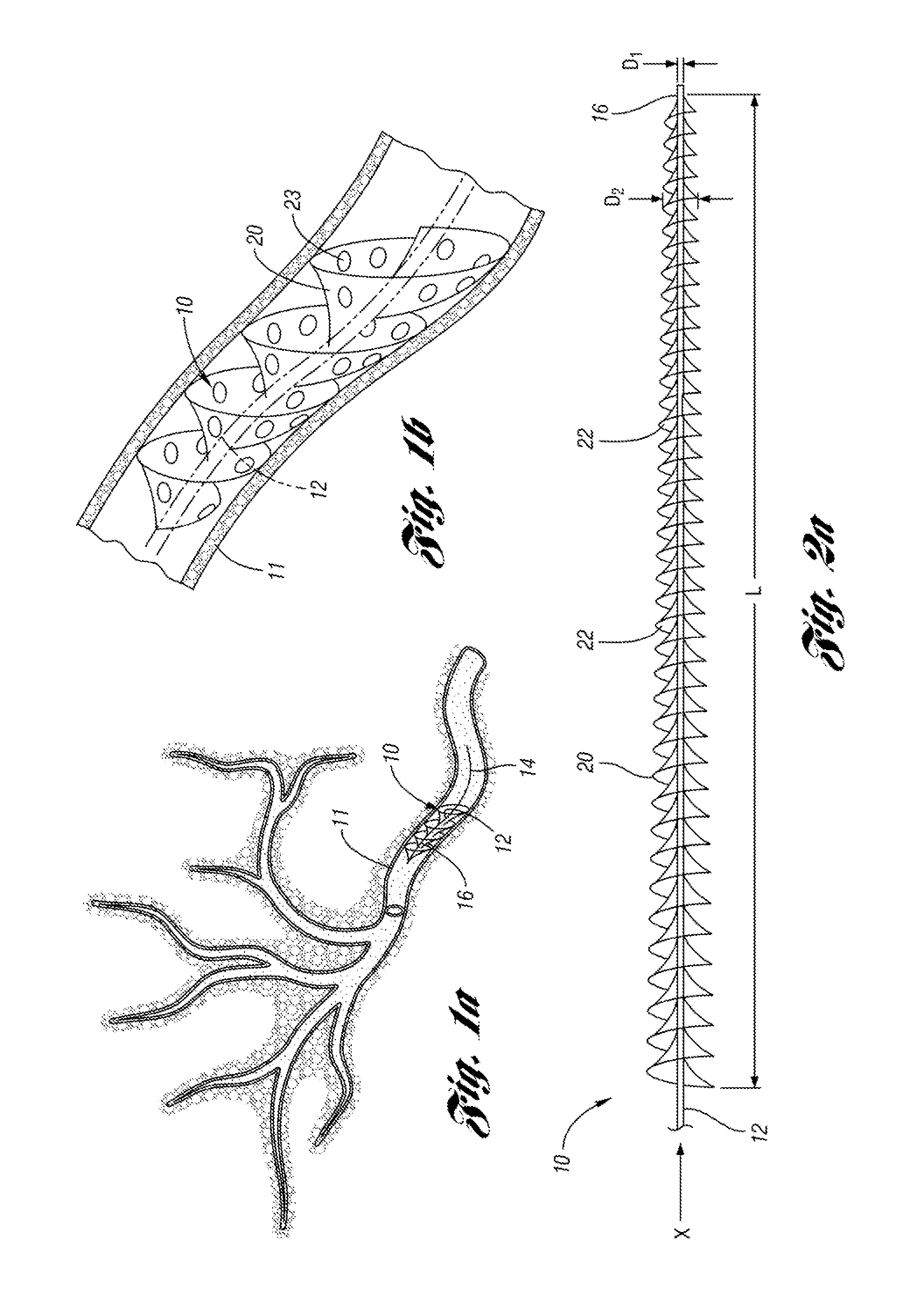
An embolic protection device for capturing emboli during treatment of a stenotic lesion in a body vessel is disclosed. The device comprises an elongated shaft having a proximal portion and a distal portion extending from the proximal portion. The device further comprises an expandable filter formed helically about the distal portion of the elongated shaft. The expandable filter is configured to helically close defining a collapsed state for delivery and retrievable of the device. The expandable filter is configured to helically open defining an expanded state for capturing emboli during treatment of the stenotic lesion in the body vessel. The expandable filter has at least one filter portion helically extending from the elongated shaft at a predetermined angle defining an opening when the filter is in the expanded state.
Owner:COOK MEDICAL TECH LLC
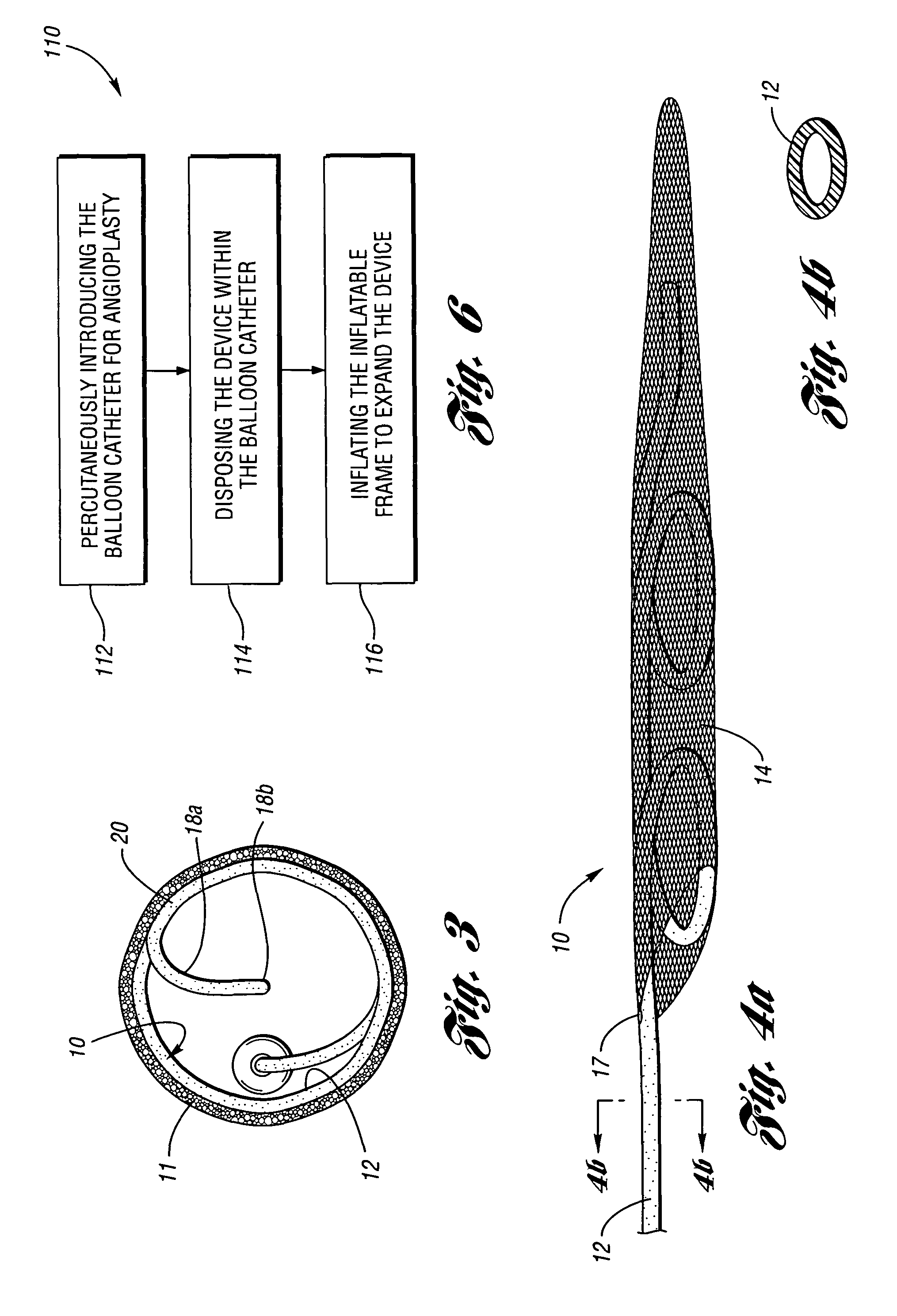
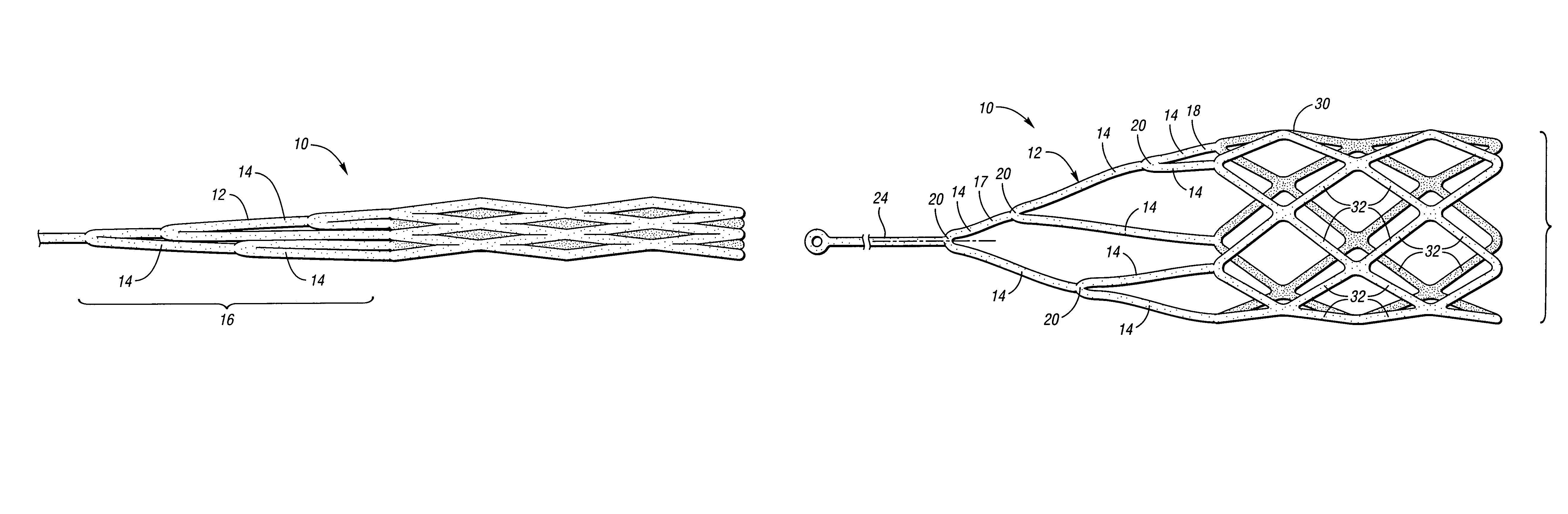
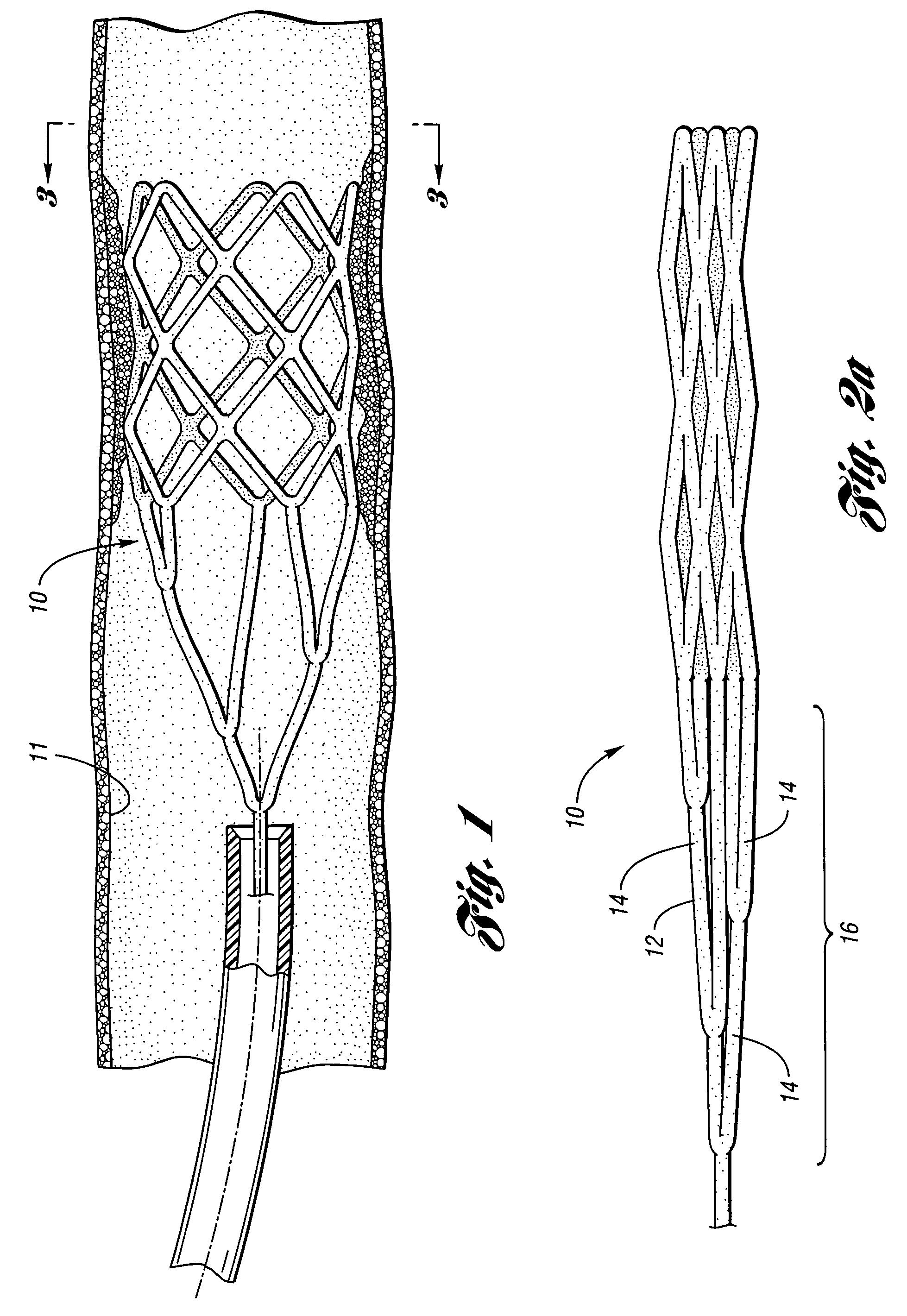
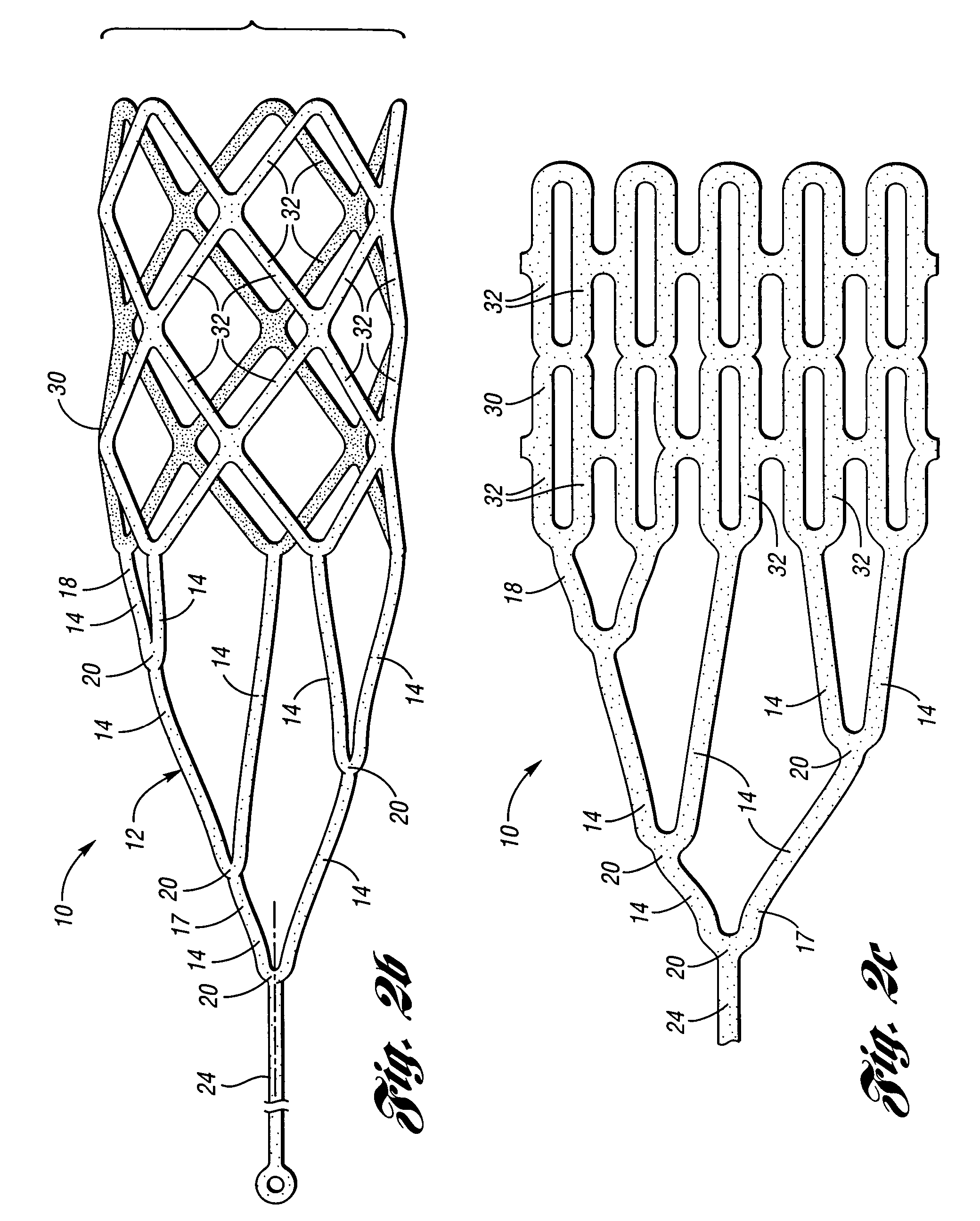
Embolic protection device having inflatable frame
An embolic protection device for capturing emboli during treatment of a stenotic lesion in a body vessel is provided. The device includes an inflatable frame having a deployed state and an undeployed state and a filter attached to the inflatable frame for capturing emboli. The inflatable frame includes a distal portion having a predetermined shape and extending freely to a closed distal end when the inflatable frame is in the deployed state. The filter includes a lip attached to the inflatable frame to define an open end of the filter when the inflatable frame is in the deployed state, a body extending from the lip and disposed about the inflatable frame, and a closed tail configured to capture the emboli during treatment of the stenotic lesion.
Owner:COOK MEDICAL TECH LLC
Retrievable device having a reticulation portion with staggered struts
A retrievable device for treatment of a stenotic lesion in a body vessel is disclosed. The device comprises a reticulation portion including a plurality of struts connected together in a singly staggered configuration distally along a longitudinal axis. The plurality of struts of the reticulation portion is configured to fold along the longitudinal axis defining a collapsed state of the device for retrieval. The device further includes an expandable body distally extending from the reticulation portion along an outer diameter for treatment of the stenotic lesion. The expandable body is configured to expand in the open state and collapsed in the collapsed state of the reticulation portion for retrieval. The device further comprises a retrieval stem extending proximally from the reticulation portion for retrieval of the device in the collapsed state.
Owner:COOK MEDICAL TECH LLC
Rotational atherectomy device with counterweighting
The invention provides a rotational atherectomy device having a flexible, elongated, rotatable drive shaft with an abrasive section comprising an enlarged diameter section of the drive shaft or, alternatively, a solid abrasive crown which may be attached to the drive shaft. The device further comprises a proximal and / or a distal counterweight attached to the drive shaft, spaced from the abrasive section wherein each counterweight has its center of mass offset from the longitudinal axis of the drive shaft to stimulate orbital motion by the abrasive section. When placed within an artery against stenotic tissue and rotated at sufficiently high speeds (e.g., in the range of about 20,000 rpm to about 200,000 rpm) the orbiting nature of the abrasive section causes such section to rotate as to open the stenotic lesion to a diameter substantially larger than the resting outer diameter of the abrasive section.
Owner:CARDIOVASCULAR SYST INC
Embolic protection device having a filter
InactiveUS20070100372A1Minimizes restricted flowEasy retrievalSurgeryDilatorsStenotic lesionEmbolic Protection Devices
An embolic protection device for capturing emboli during treatment of a stenotic lesion in a body vessel is disclosed. In one example, the device comprises a filter and a filter portion made of an extracellular matrix circumferentially attached to the filter. The filter comprises a plurality of struts having first ends attached together at a center portion along a longitudinal axis. Each strut has an arcuate segment extending from the first end to a second end. The struts are configured to move between an expanded state for engaging with the body vessel and a collapsed state for filter retrieval or delivery. The filter portion is configured for allowing blood to flow therethrough and for capturing emboli when the filter is in the expanded state.
Owner:COOK INC
Embolic protection device having inflatable frame
An embolic protection device for capturing emboli during treatment of a stenotic lesion in a body vessel is provided. The device includes an inflatable frame having a deployed state and an undeployed state and a filter attached to the inflatable frame for capturing emboli. The inflatable frame includes a distal portion having a predetermined shape and extending freely to a closed distal end when the inflatable frame is in the deployed state. The filter includes a lip attached to the inflatable frame to define an open end of the filter when the inflatable frame is in the deployed state, a body extending from the lip and disposed about the inflatable frame, and a closed tail configured to capture the emboli during treatment of the stenotic lesion.
Owner:COOK MEDICAL TECH LLC
Eccentric abrading head for high-speed rotational atherectomy devices
ActiveUS8597313B2Improve abilitiesMinimal traumaCannulasExcision instrumentsStenotic lesionRotational axis
Owner:CARDIOVASCULAR SYST INC
Rotational atherectomy device with a system of eccentric abrading heads
The invention provides a rotational atherectomy device having, in various embodiments, a flexible, elongated, rotatable drive shaft with a system of eccentric abrading heads attached thereto. At least part of the eccentric enlarged abrading heads in the system have a tissue removing surface—the abrading heads may be at least partially hollow. Preferably the abrading heads have centers of mass spaced radially from the rotational axis of the drive shaft, facilitating the ability of the system of abrading heads to work together to open the stenotic lesion to a diameter larger than the outer resting diameter of the abrading heads when operated at high speeds. Therefore, certain embodiments comprise a system having unbalanced centers of mass to not only stimulate greater rotational diameters but also arranged in a manner whereby a debris-removing augering effect occurs. Other embodiments may comprise systems having abrading heads with balanced centers of mass.
Owner:CARDIOVASCULAR SYST INC
Angioplasty cutting device and method for treating a stenotic lesion in a body vessel
ActiveUS20070106215A1Simple and efficient and cost-effectiveEfficient and effectiveBalloon catheterCannulasStenotic lesionBlood vessel
An integrally formed angioplasty cutting device for balloon angioplasty of a stenotic lesion in a body vessel. The device comprises a distal collar and a proximal collar. The device further comprises at least one strut integrally formed with the distal collar and the proximal collar. At least one of the collars has a slot formed therethrough defining a C-shaped configuration. The strut is configured to be disposed at the stenotic lesion to engage the stenotic lesion for dilatation of the body vessel during angioplasty.
Owner:COOK MEDICAL TECH LLC
Rotational atherectomy device with counterweighting
The invention provides a rotational atherectomy device having a flexible, elongated, rotatable drive shaft with an abrasive section comprising an enlarged diameter section of the drive shaft or, alternatively, a solid abrasive crown which may be attached to the drive shaft. The device further comprises a proximal and / or a distal counterweight attached to the drive shaft, spaced from the abrasive section wherein each counterweight has its center of mass offset from the longitudinal axis of the drive shaft to stimulate orbital motion by the abrasive section. When placed within an artery against stenotic tissue and rotated at sufficiently high speeds (e.g., in the range of about 20,000 rpm to about 200,000 rpm) the orbiting nature of the abrasive section causes such section to rotate as to open the stenotic lesion to a diameter substantially larger than the resting outer diameter of the abrasive section.
Owner:CARDIOVASCULAR SYST INC
Rotational atherectomy segmented abrading head and method to improve abrading efficiency
The invention provides a rotational atherectomy system, device and method having, in various embodiments, a flexible, elongated, rotatable drive shaft comprising an eccentric abrading head comprising at least one eccentric abrading cylindrical segments attached to the drive shaft and in spaced proximity with proximal and a distal conical segments. Each individual abrading segment, comprises a first tissue removing surface, typically an abrasive coating on the outer surface, that is designed to abrade calcified, hard tissue and abrasive coating on the leading and trailing surfaces designed to abrade non-calcified, soft tissue. Each abrading segment, as well as the abrading head comprising the collective segments, has a center of mass spaced radially from the rotational axis of the drive shaft, facilitating the ability of the device to open the stenotic lesion to a diameter larger than the outer diameter of the enlarged abrading head when operated at high speeds.
Owner:CARDIOVASCULAR SYST INC
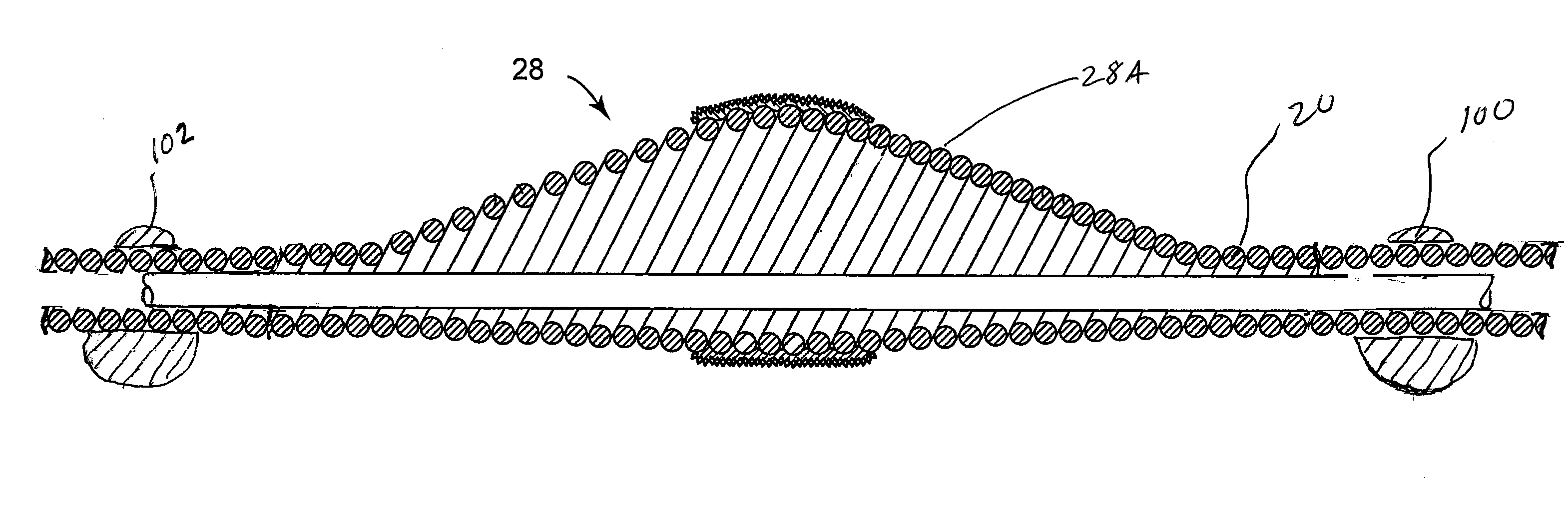
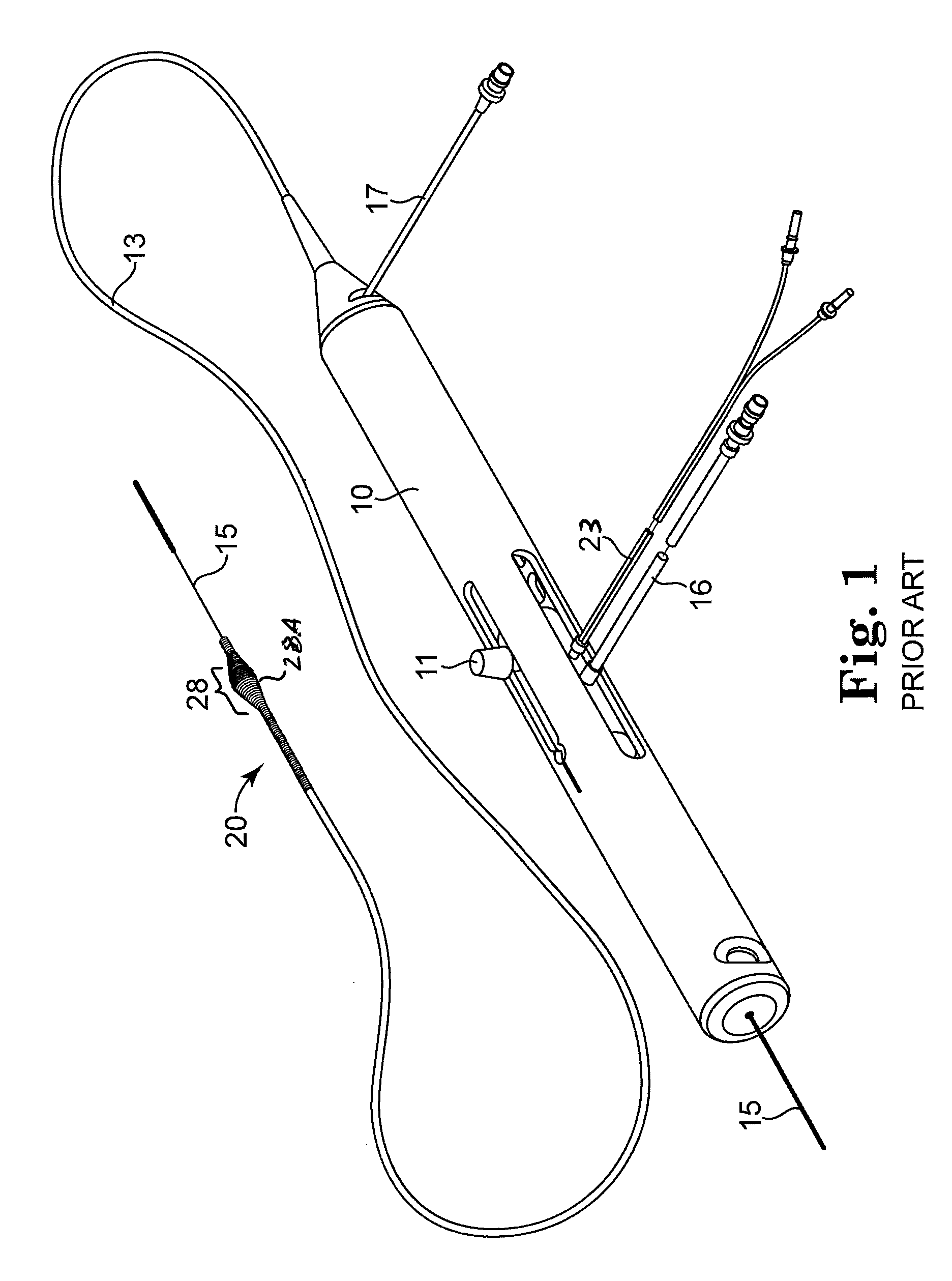
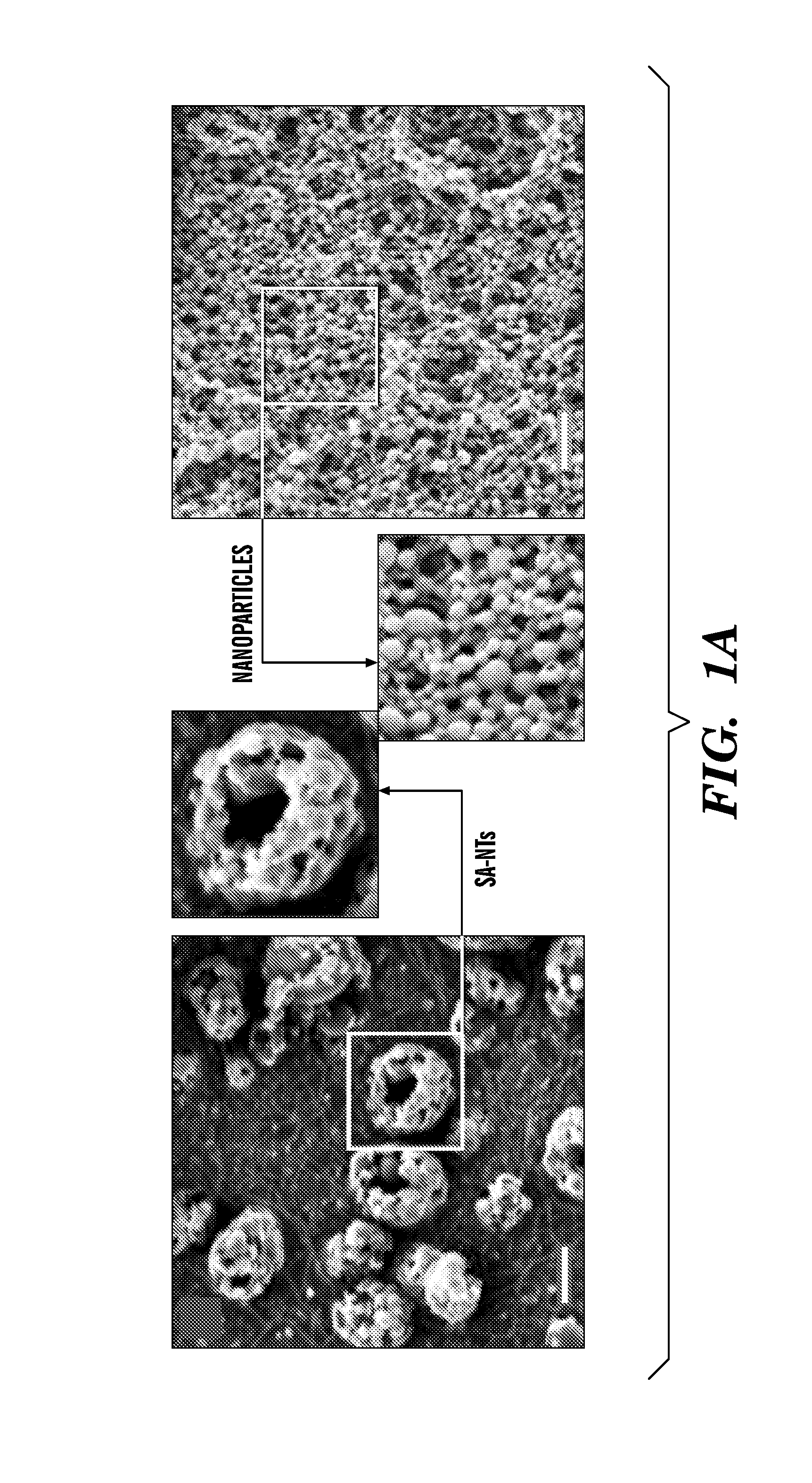
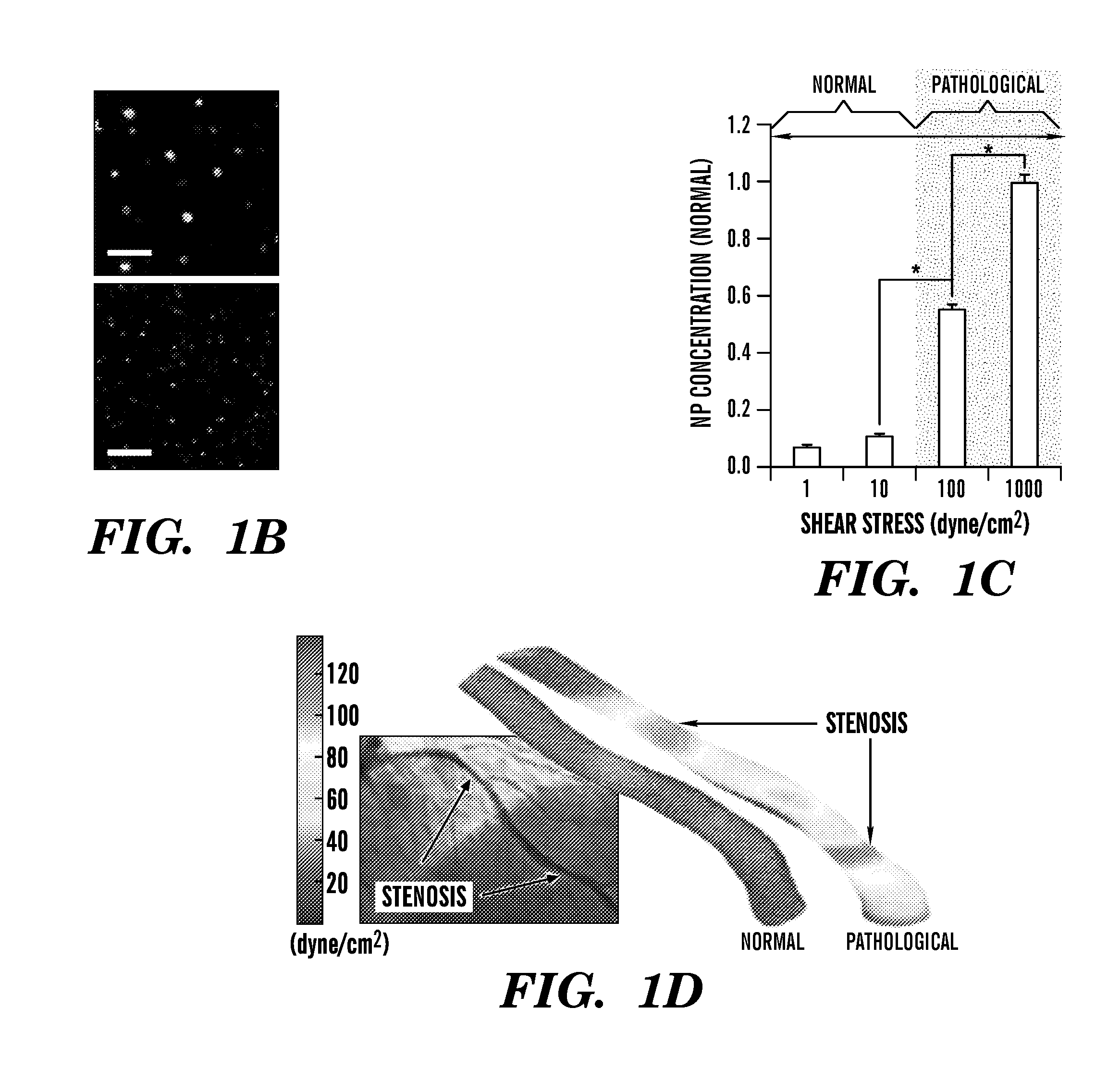
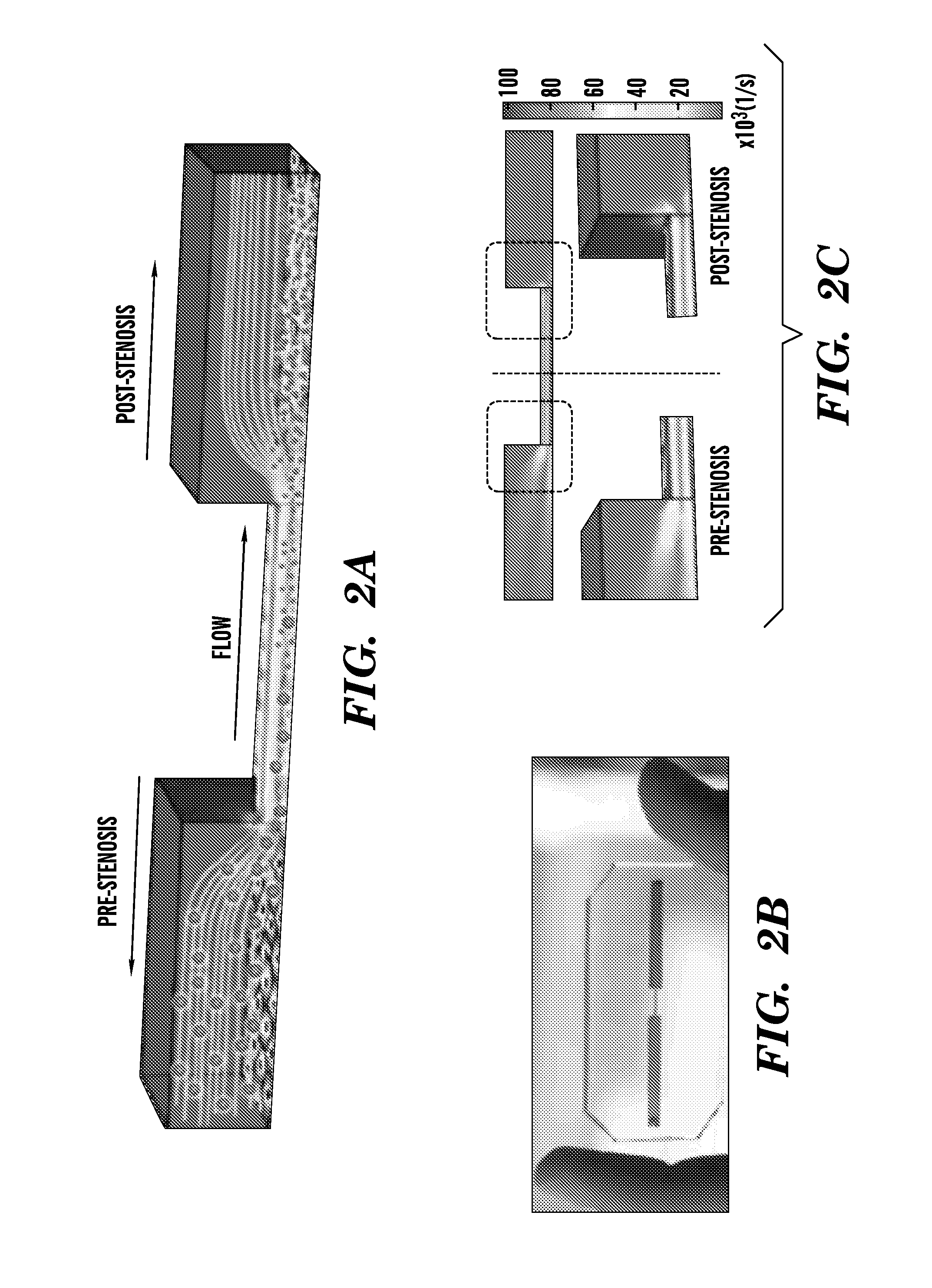
Nanotherapeutics for drug targeting
InactiveUS20150147276A1Antibacterial agentsUltrasonic/sonic/infrasonic diagnosticsStenotic lesionTarget control
The invention provides compositions and methods for targeted controlled drug release. The compositions and methods can be used for treating or imaging vascular stenosis, stenotic lesions, occluded lumens, embolic phenomena, thrombotic disorders and internal hemorrhage.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
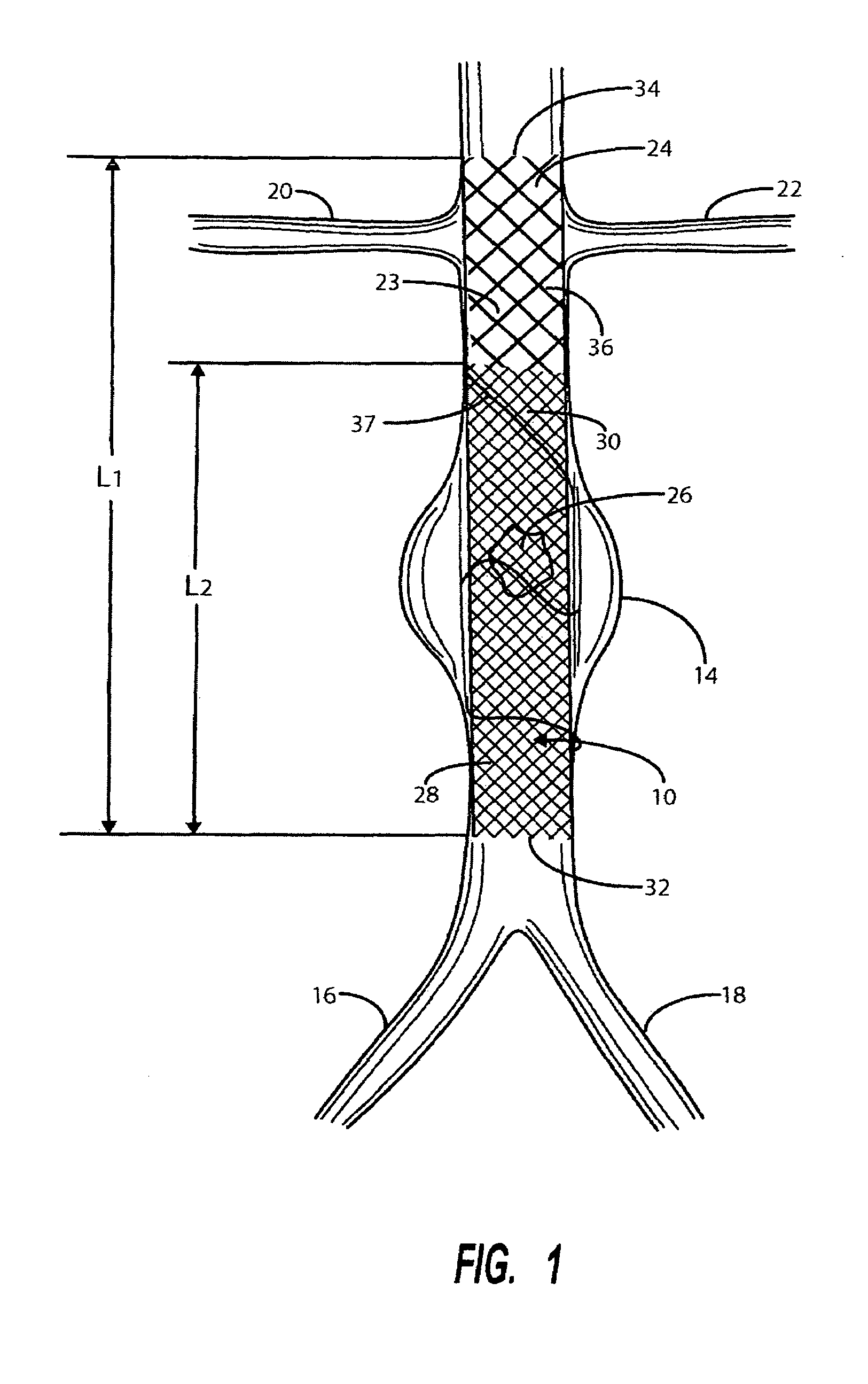
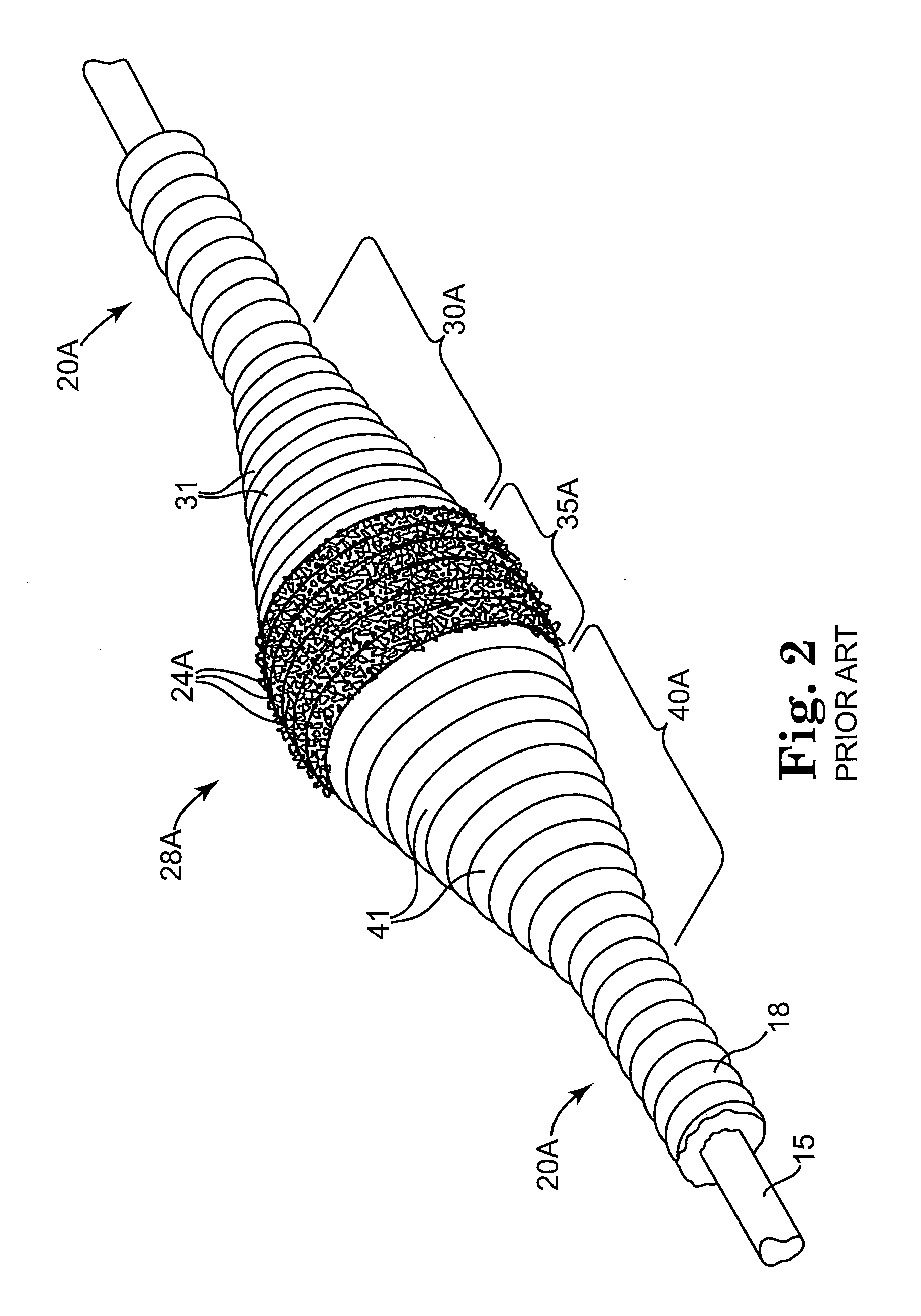
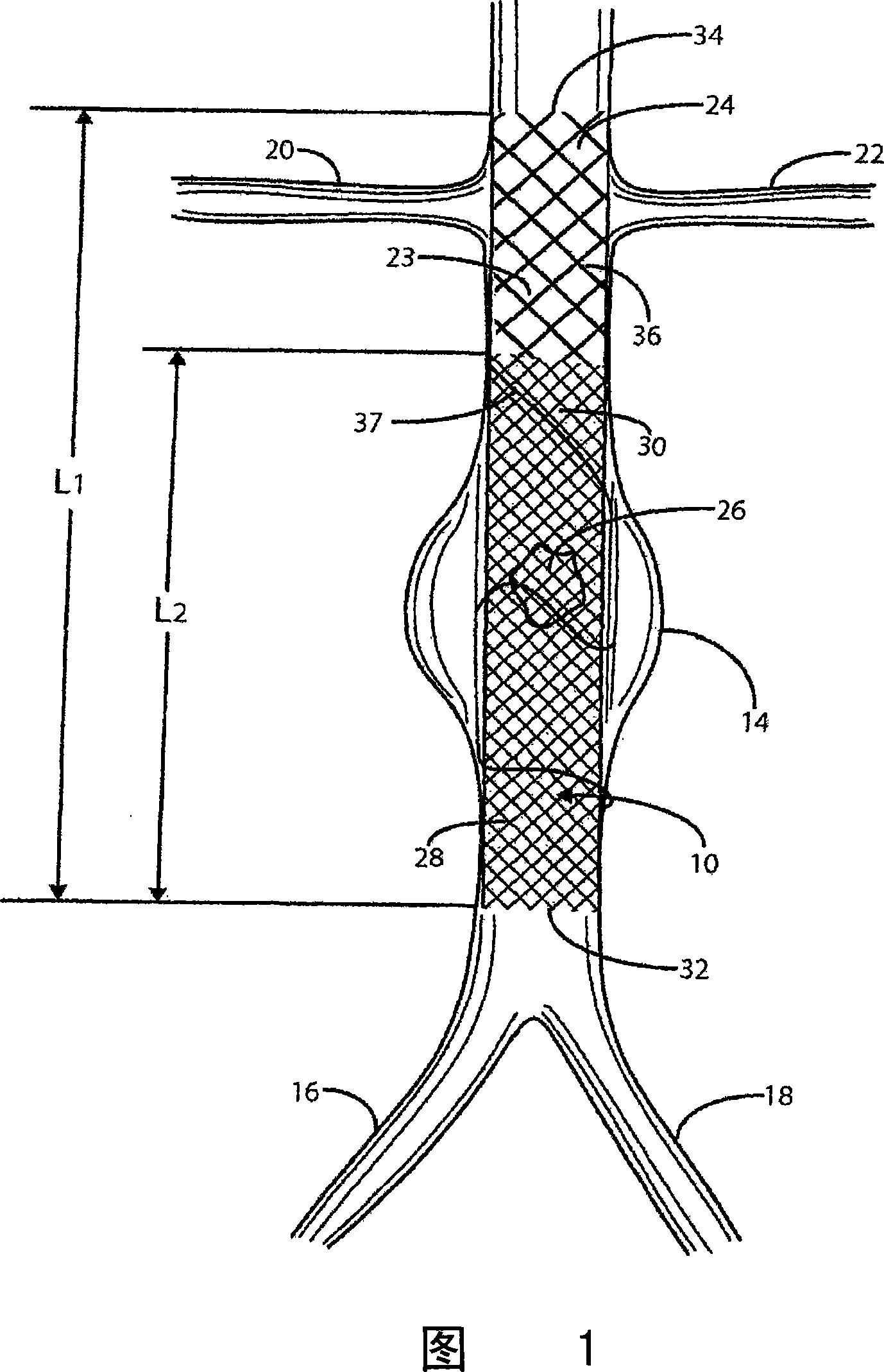
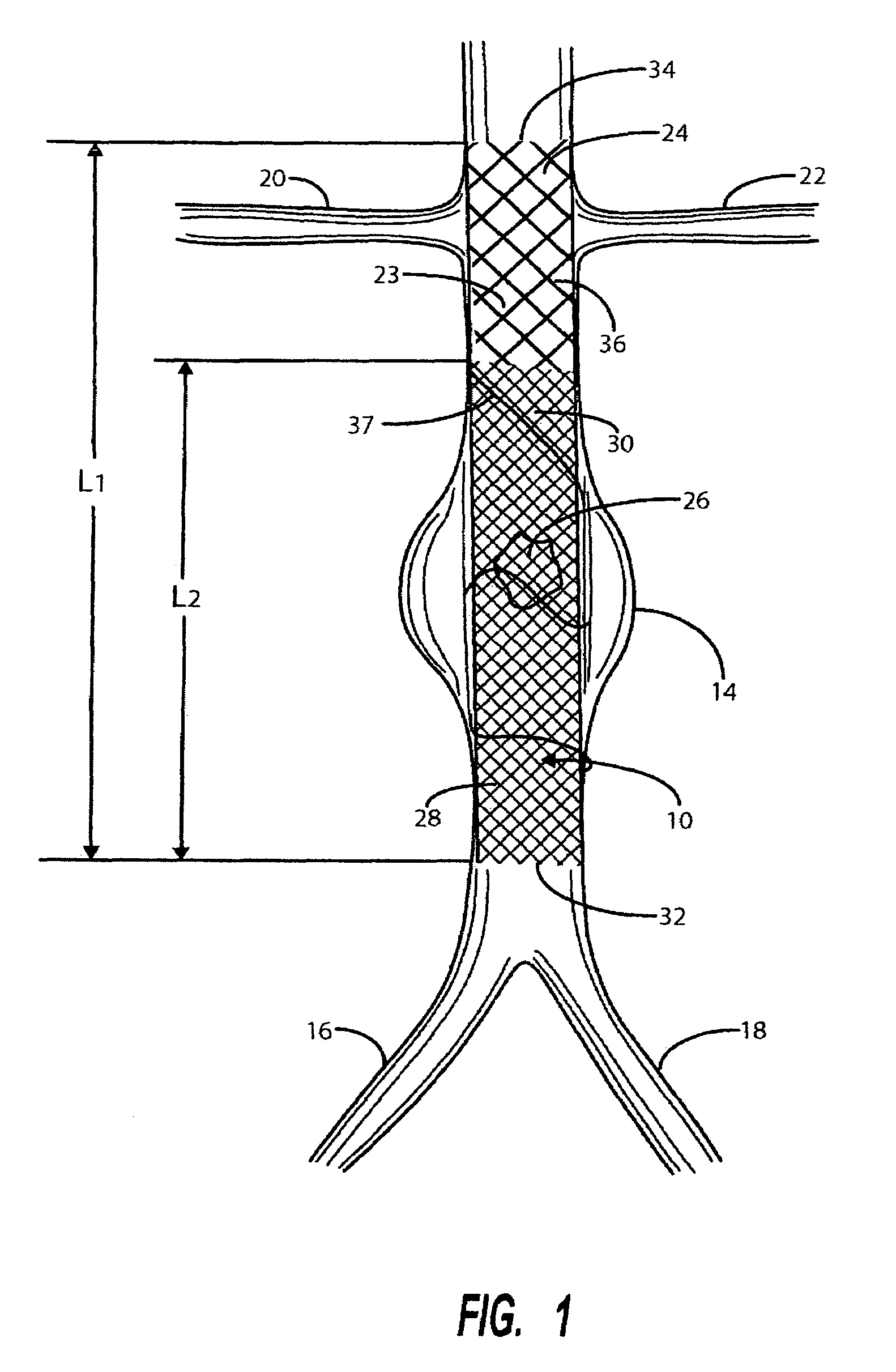
Intravascular deliverable stent for reinforcement of vascular abnormalities
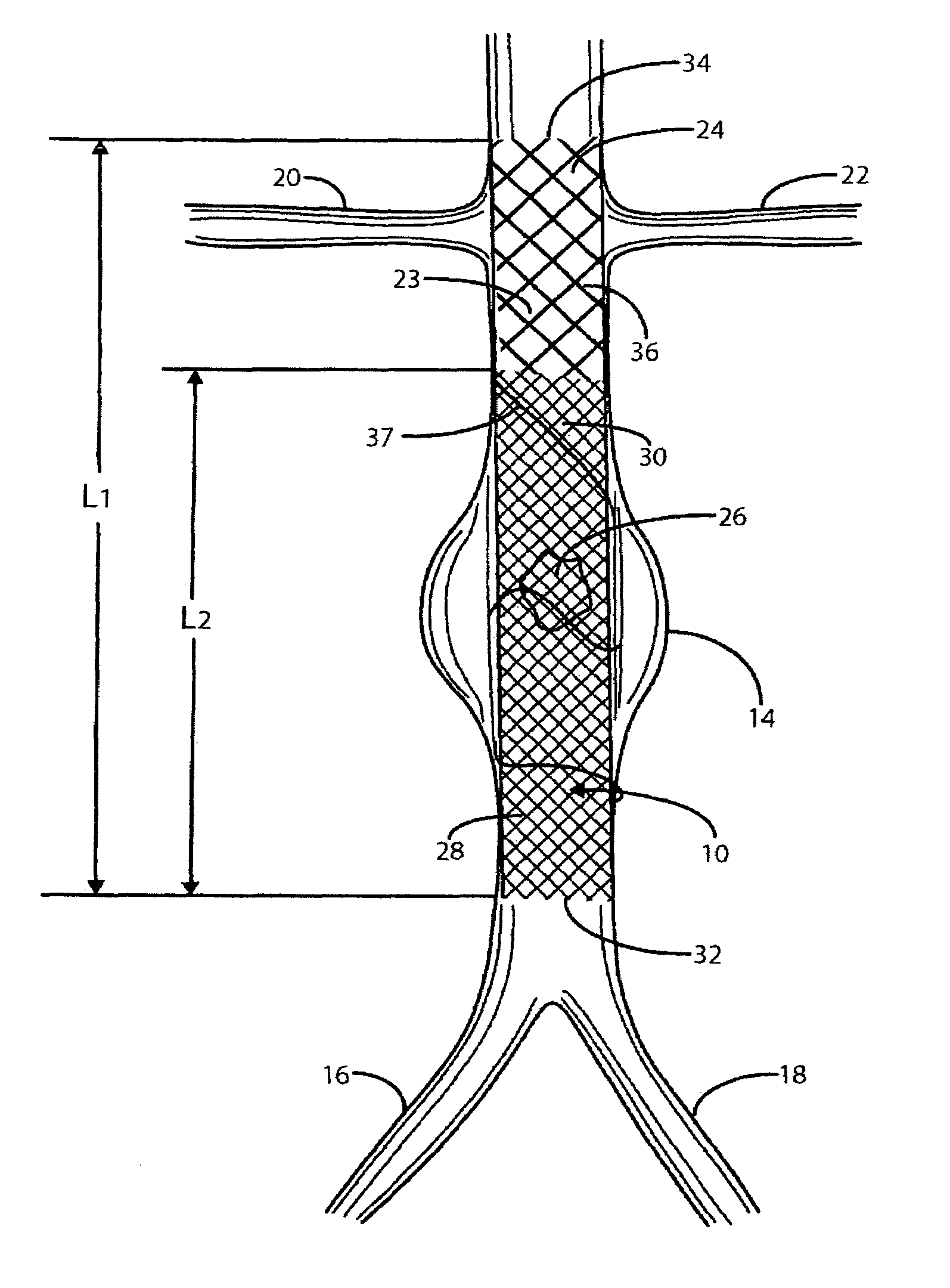
A catheter deliverable stent / graft especially designed to be used in a minimally invasive surgical procedure for treating a variety of vascular conditions such as aneurysms, stenotic lesions and saphenous vein grafts, comprises an innermost tubular structure and at least one further tubular member in coaxial arrangement. In one embodiment, the innermost tubular structure is of a length (L 1 ) and is formed by braiding a relatively few strands of highly elastic metallic alloy. The pick and pitch of the braid are such as to provide relative large fenestrations in the tubular wall that permit blood flow through the wall and provide the primary radial support structure. A portion of the innermost tubular structure of a length (L 1 ) is surrounded by a further braided tubular structure having relatively many strands that substantially inhibit blood flow through the fenestrations of the innermost tubular structure.
Owner:AGA MEDICAL CORP MS US
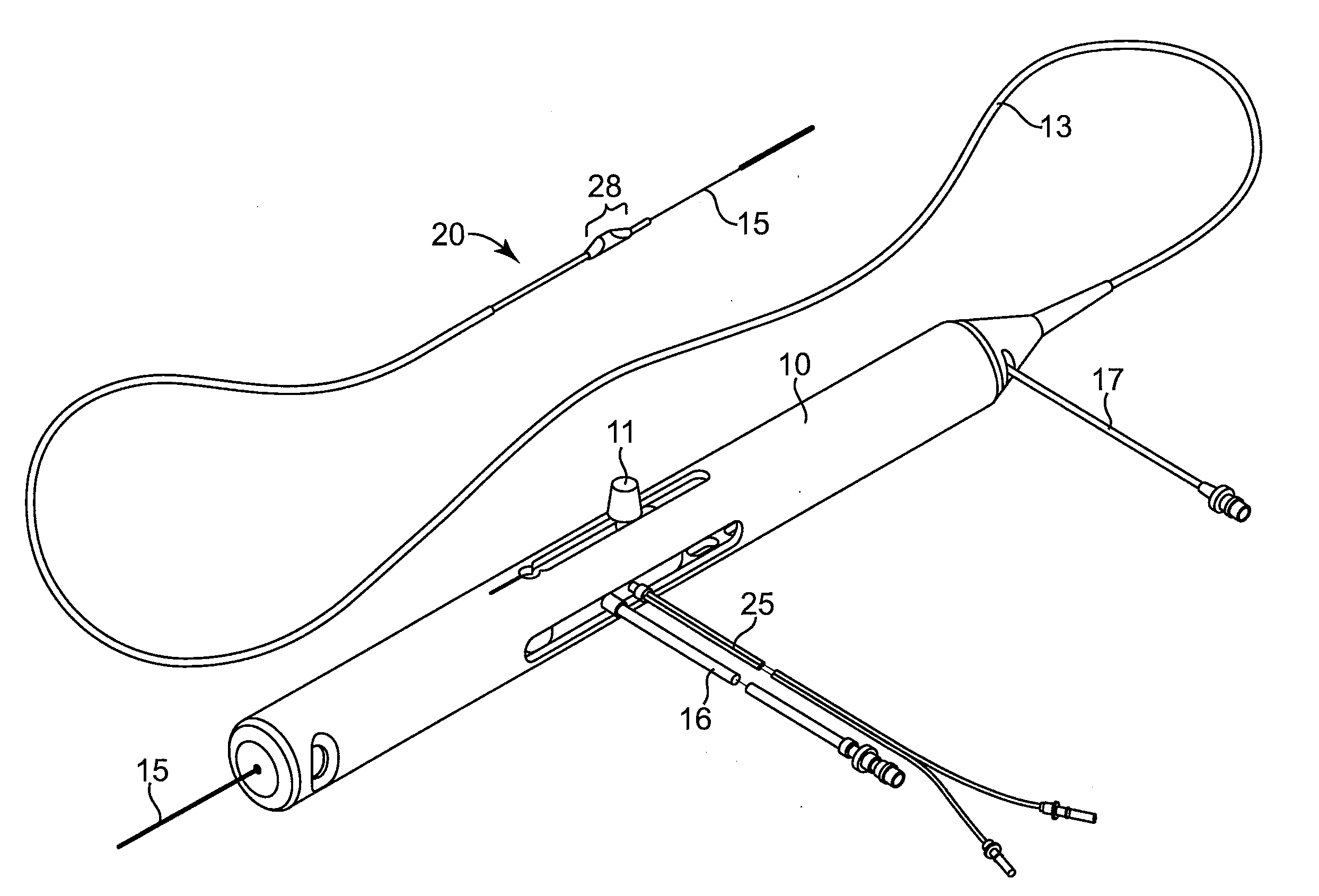
Physiological sensor delivery device and method
Owner:ACIST MEDICAL SYST
Angioplasty cutting device and method for treating a stenotic lesion in a body vessel
InactiveUS20060173487A1Increase likelihoodSimple and efficient and cost-effectiveBalloon catheterDilatorsStenotic lesionSurgery
An angioplasty cutting device for balloon angioplasty of a stenotic lesion in a body vessel. The device comprises a distal ring and a proximal ring. The device further comprises at least one strut attached to the distal ring and proximally extending to the proximal ring. The strut is configured to be disposed at the stenotic lesion to engage the stenotic lesion for dilatation of the body vessel during angioplasty. The strut extending from the proximal ring a predetermined length for delivery and retrieval of the device.
Owner:COOK INC
Devices, systems, and methods for removing stenotic lesions from vessels
The present application provides various devices, systems, and methods for removing stenotic lesions from vessels. In at least one embodiment of an exemplary device, the device comprises at least one sizing portion and at least one treatment portion. Such a device may be useful to, for example, remove a stenotic lesion from a vessel by positioning the device within a vessel lumen, operating the sizing portion to obtain luminal size parameter data, operating the treatment portion at a location within the vessel lumen at or near a stenotic lesion, and ceasing operation of the treatment portion when the luminal size parameter data indicates a preferred luminal size parameter.
Owner:3DT HLDG
Intravascular deliverable stent for reinforcement of vascular abnormalities
InactiveUS8778008B2Reduce overall outer diameterAvoid interactionStentsCatheterVascular Skin TumorSaphenous veins
A catheter deliverable stent / graft especially designed to be used in a minimally invasive surgical procedure for treating a variety of vascular conditions such as aneurysms, stenotic lesions and saphenous vein grafts, comprises an innermost tubular structure and at least one further tubular member in coaxial arrangement. In one embodiment, the innermost tubular structure is of a length (L1) and is formed by braiding a relatively few strands of highly elastic metallic alloy. The pick and pitch of the braid are such as to provide relative large fenestrations in the tubular wall that permit blood flow through the wall and provide the primary radial support structure. A portion of the innermost tubular structure of a length L1 is surrounded by a further braided tubular structure having relatively many strands that substantially inhibit blood flow through the fenestrations of the innermost tubular structure. The composite structure can be stretched to reduce the outer diameter of the stent / graft, allowing it to be drawn into a lumen of a delivery catheter. The catheter can then be advanced through the vascular system to the site of treatment and then released, allowing it to self-expand against the vessel wall. Various optional embodiments are disclosed that allow one skilled in the art to tailor the design to the specific application.
Owner:ST JUDE MEDICAL CARDILOGY DIV INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com