Epitope of systemic lupus erythematosus and application thereof
A lupus erythematosus and antigenic epitope technology, applied in the field of immunological diagnosis, can solve the problems of unclear pathogenesis, self-antigen detection antibody, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Example 1 SLE patient serum total IgG preparation and purification
[0021] Take an appropriate amount of Protein A resin suspension (product of GE Company), put it into the chromatography column, and wash the equilibrium chromatography column with 10 times the column volume of PBS buffer. After the serum of patients with systemic lupus erythematosus was centrifuged at high speed, 1 / 10 volume of 10×PBS buffer solution was added to mix, and the pH and ion concentration were adjusted, then slowly added to the chromatography column. Wash with more than 10 times the column volume of PBS until no protein is detected in the effluent. Add 6 times column volume of 0.1 M glycine (pH 3.0) to elute, collect the flow-through, and neutralize with 1 / 10 volume of 1M Tris-HCl (pH 9.0). Dialyze the obtained antibody against PBS, add 50% glycerol after quantifying the antibody concentration, aliquot and store at -20°C.
Embodiment 2
[0022] Example 2 Phage display peptide library screening for SLE-specific antibody-binding peptides
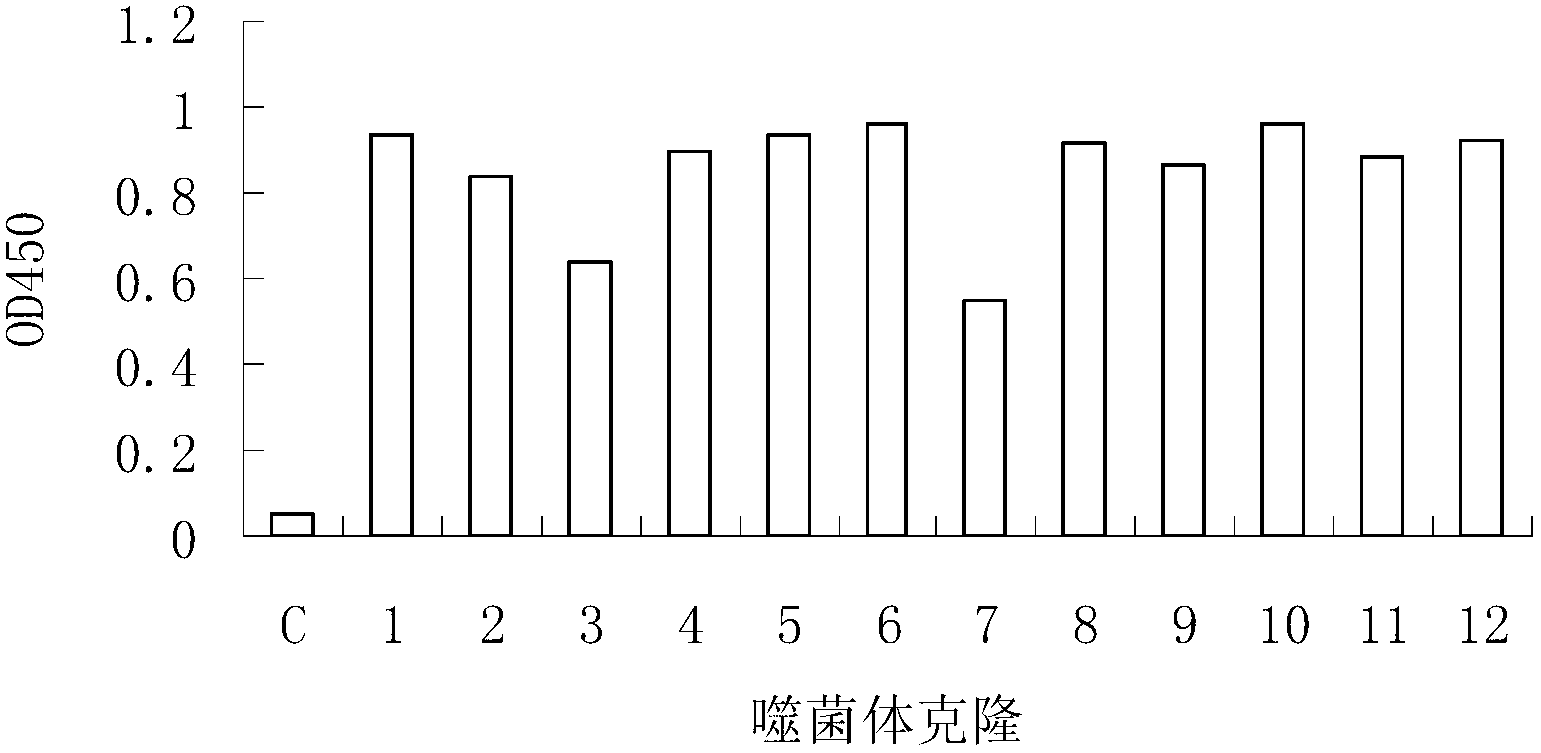
[0023] Ph.D.-12 peptide library kit was purchased from NEB Company, with a library capacity of 2.7×109 and a titer of 1.5×1013 / μl. Escherichia coli ER2537 was used as the host bacteria of the peptide library. For the screening process, refer to the instruction manual of the phage display peptide library. . Each round of screening was coated with total IgG in the serum of SLE patients (10 μg per well), and 1×1011 phage were injected into each well. The degree of phage enrichment was calculated by "input / output ratio". After three rounds of screening, positive clones were selected for sequencing. The specific binding of positive phage clones to total IgG antibodies was determined by ELISA. Total IgG antibody (10 μg per well) was coated on a 96-well plate (Nunc Company) overnight at 4 °C. Pour off the unbound antibody and block with 3% BSA at 37°C for 1 hour. Put 1×109 phage...
Embodiment 3
[0024] Example 3 SLEP and SLE patient serum IgG binding detection
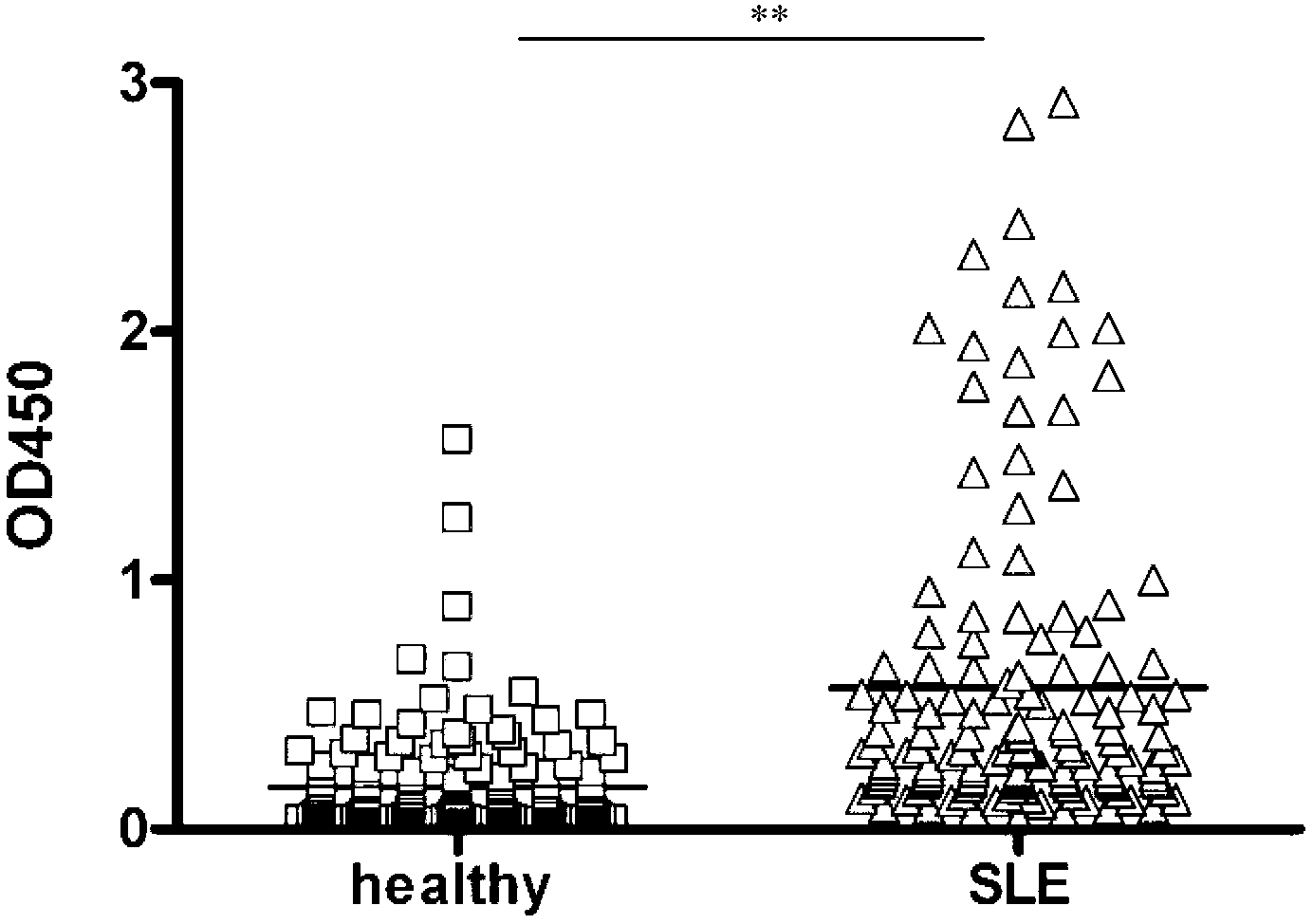
[0025] The synthetic peptide SLEP (0.5 μg per well) was coated on a 96-well solid microtiter plate (Nunc Company) overnight at 4 °C. Unbound peptides were poured off and blocked with 3% bovine serum albumin (BSA) at 37°C for 1 hour. Serum samples from SLE patients or healthy individuals were diluted 1:400, added to different wells, and incubated at 37°C for 1 hour. Wash the plate with washing solution (PBS-0.05% Tween 20) 5 times for a total of 3 minutes. 1:1000 dilution of HRP-labeled goat anti-human IgG was incubated at 37°C for 1 hour. The washing method was the same as above, and the bound anti-M13 monoclonal antibody was detected with tetramethylbenzidine (TMB, Sigma) as the substrate, and the light absorption was detected at 450 nm.
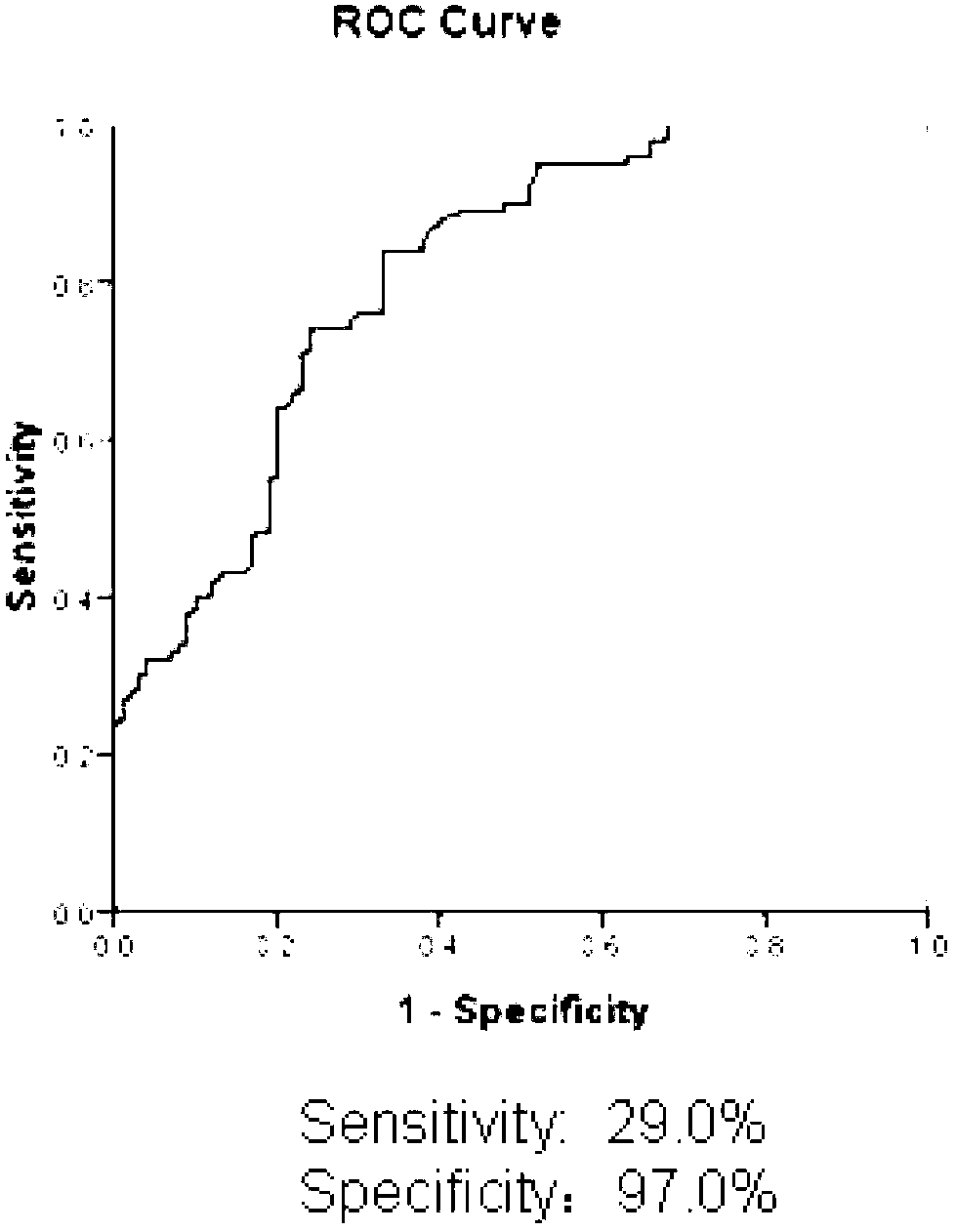
[0026] see results figure 2 , the level of SLEP antibody in the serum of patients was significantly higher than that of healthy people. Statistical analysis was perfor...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Absorbance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com