i(Ehrlichia canis) 120-KDa immunodominant antigenic protein and gene
A technology for Ehrlichia and antigens, used in the production of recombinant proteins, preparations for rickettsial diseases and Ehrlichia, in the field of recombinant proteins, which can solve the problems of cloning and characterizing genes without immunoreactivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0100] Ehrlichia
[0101]The Oklahoma strain of E. canis was provided by Dr. Jacqueline Dawson (Centers of Disease Control, Atlanta, GA). Dr. Edward B. Breitschwerdt (College of Veterinary Medicine, North Calorina State University, Raleigh, NC) provided the Florida strain of E. canis, and three North Carolina isolates (Demon, DJ, and Jake). Dr. R. E. Corstvet (Louisiana State University, Baton Rouge, Louisiana) provided the Louisiana strain of E. canis. Ehrlichia were grown in DH82 cells (a canine macrophage-like cell line, Dawson JE, 1991). When 100% of the cells were infected with Ehrlichia, the DH82 cells were collected with a cell scraper. Centrifuge cells at 17,400xg for 20 minutes. The pellet was pulverized twice on ice with a Braun-Sonic2000 ultrasonic generator at 40W. Cell lysates were added to a step gradient of 42%-36%-30% renografin and centrifuged at 80,000 xg for 60 minutes. Ehrlichia in heavy and light bands were collected (Weiss E, 1975) and washed with ...
Embodiment 2
[0103] DNA preparation
[0104] Genomic DNA of E. canis was prepared from renografin density gradient purified E. canis using the IsoQuick Nucleic Acid Extraction Kit (ORCA Research Inc., Bothell, WA) according to the manufacturer's instructions. Plasmid DNA was purified using a high-purity plasmid isolation kit (Boehringer Mannheim Corp., Indianapolis, IN). PCR products were purified using the QIAquick PCR Purification Kit (QIAGEN Inc., Santa Clarita, CA).
Embodiment 3
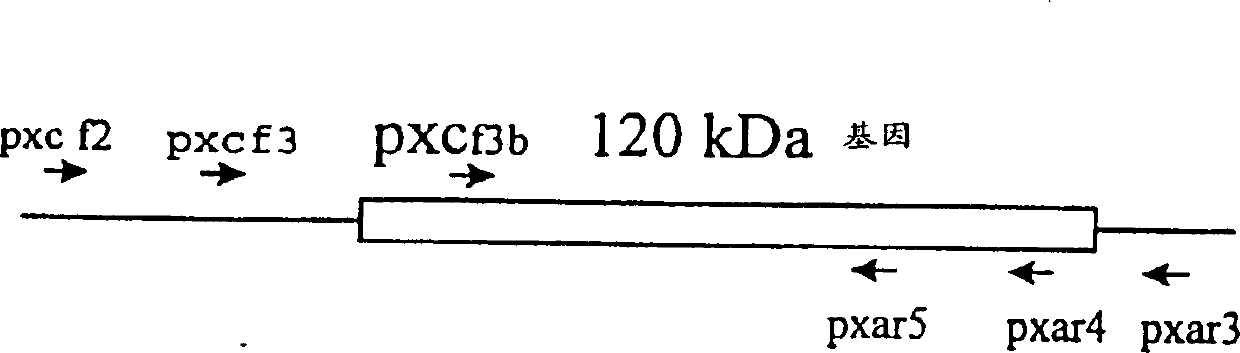
[0106] PCR Amplification of 120kDa Protein Gene of Ehrlichia canis
[0107] Primers (Yu, X-J1996) were designed based on the DNA sequence of the E. chaffeensis 120-kDa protein gene (SEQ ID NO: 1-6, FIG. 1 ). Using PCR, the E. canis 120 kDa protein gene was amplified through 30 rounds of 94°C for 30 seconds, 52°C for 1 minute and 72°C for 2 minutes. The PCR amplified product was cloned into pCR2.1 TA cloning vector (Invitrogen, Carlsbad, CA).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com