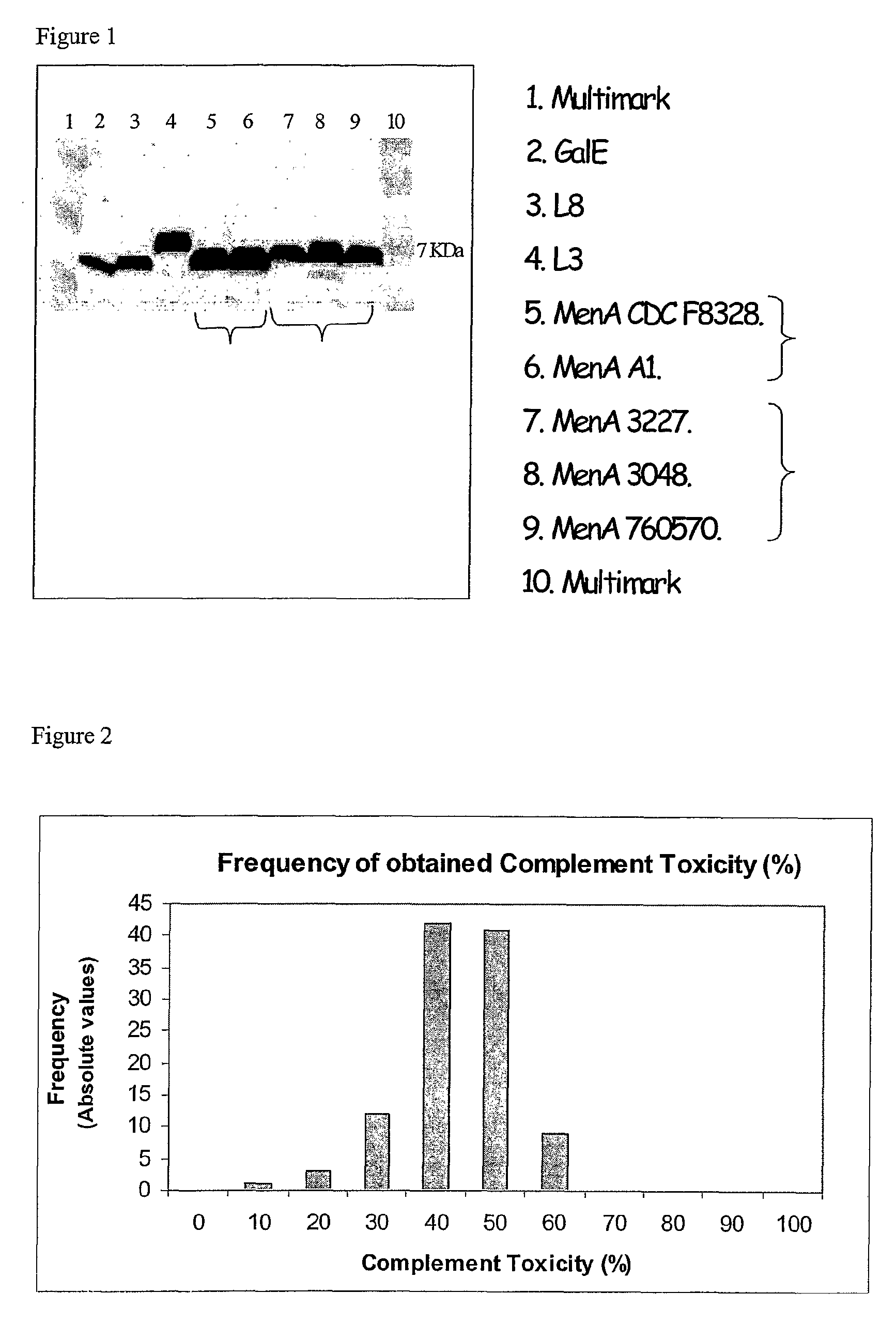
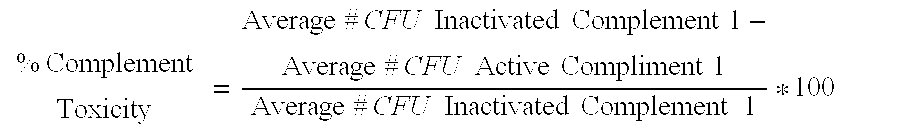
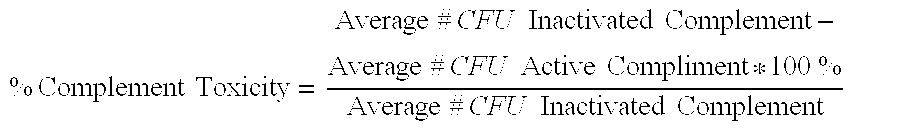Serum bactericidal assay for N. meningitidis specific antisera
a technology of n. meningitidis and specific antisera, which is applied in the field of serum bactericidal assay for n. meningitidis specific antisera, can solve the problems that the assay using the strain does not efficiently differentiate the responses pre- and post-immunization, and achieves the effect of minimizing the effect of the presen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1a
A Typical MenA SBA Assay Procedure (e.g. using the 3125 or F8238 Men A Strain as the Reference Strain)
Day Change
Day 1 1. Pre-culture of the Working Seed
[0072]Working Seed Men A 3125 [or F8238](−70° C.).[0073]Spread 50 μL of bacteria in isolation way across the following plates:[0074]3 brain heart infasion+1% horse serum (BHI) (4° C.).[0075]1 thayer Martin (TM)→selective media for Nmen with Antibiotic (4° C.). [control plate which showing N. meningitidis cells are present][0076]1 nutrient agar (NA)→detection of the contamination (4° C.). [control plate showing there is no contaminants growing—Nmen should not grow][0077]Incubate overnight or for approximately 40-42 hours at 37° C., 5% CO2 with humidity for colonies to grow.
Day 2 2. Culture of the WS on Solid Media[0078]Control the TM and NA media: verify before the test.[0079]Take about 20 colonies (well isolated) of 1 BHI petri dish.[0080]Spread these colonies across 1 BHI, repeat this operation 3 times.[0081]Incubate during 4 hours ...
example 1b
Typical Method to Calculate % Seropositivity
[0129]Carry out the method of Example 1a or 2 with the chosen reference strain with sera from at least 25 (for instance at least 50) different naïve children between the ages of 1 and 2 (when protective immunity is low in the majority of such children).
[0130]To look at the specificity of the SBA assay, sera from naïve children [i.e. who have not been vaccinated with a MenA and / or MenW vaccine (for instance a capsular saccharide conjugate)], the sera can be assessed for SBA titres achieving 50% cell killing. The % of these subjects with sera achieving a reciprocal titer equal to or more than 8 in the SBA test is defined as % seropositivity.
[0131]% seropositivity post immunization with MenA and / or MenW vaccine can also be done in this way.
example 1c
Typical Method to Calculate % Seroconversion
[0132]Carry out the method of Example 1a or 2 with the chosen reference strain with sera from at least 25 (for instance at least 50) different children (naïve Dust prior to immunization] and one month post immunization with a unconjugated N. meningitidis ACWY capsular polysaccharide vaccine e.g. Mencevax™ from GlaxoSmithiKine Biologicals s.a.) over the age of 3 (e.g. between the ages of 3 and 5, or between the ages of 18 and 25).
[0133]To assess the sensitivity of the SBA assay, the % seroconversion against the strain is measured by assessing the % of subjects with at least a 4 fold increase in rSBA titre (to achieve 50% cell killing).
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| humidity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com