Patents
Literature
32 results about "Serotype a" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
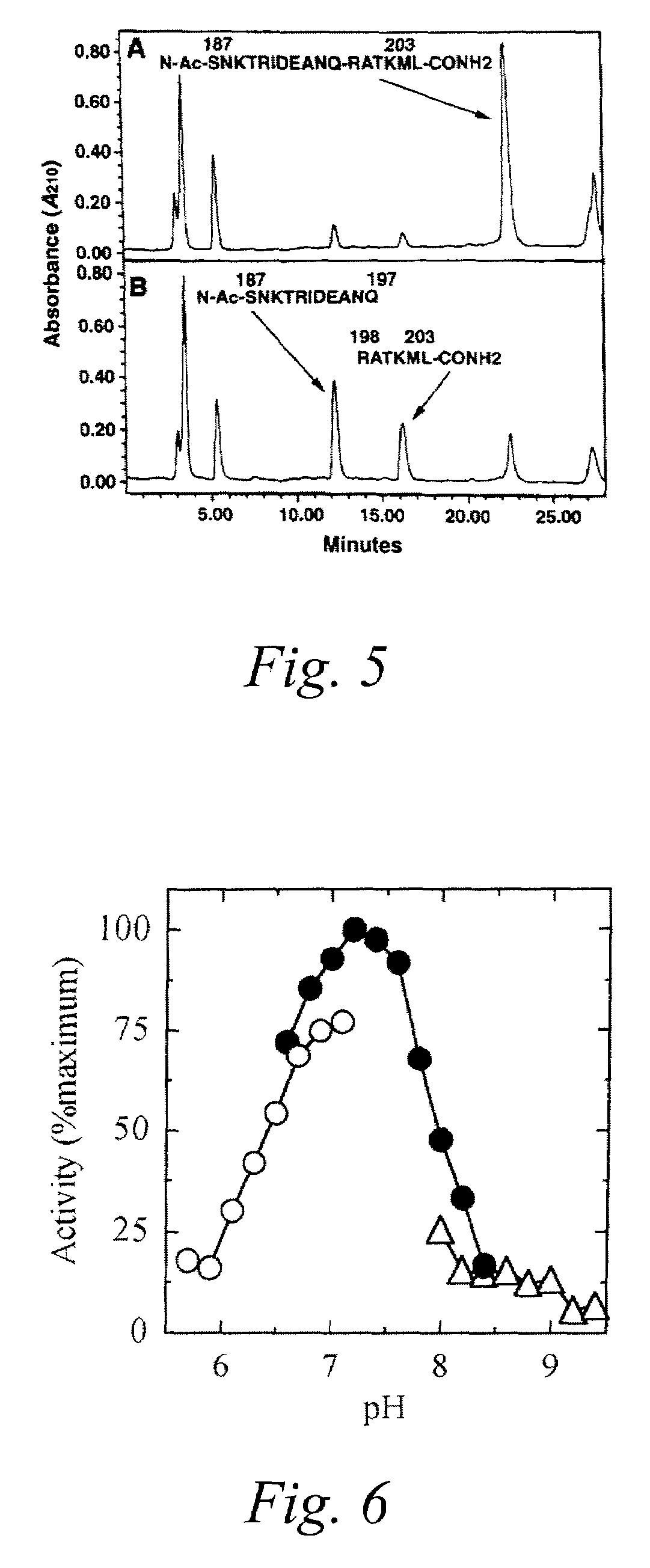
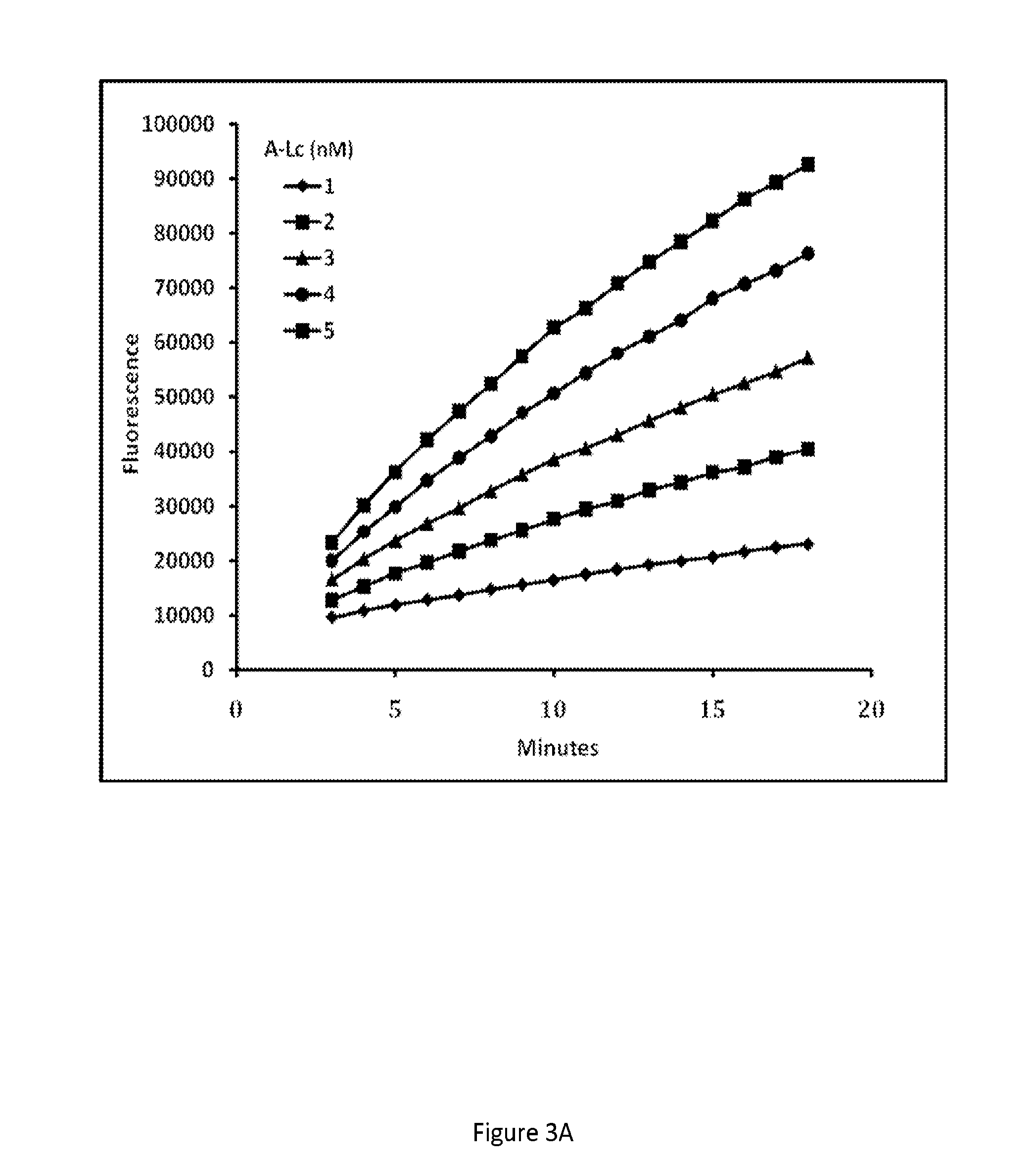
Fret protease assays for botulinum serotype A/E toxins
InactiveUS7208285B2Used to determineDecreased acceptor fluorescence intensityBacteriaSugar derivativesFluorophoreReceptor for activated C kinase 1
The present invention provides clostridial toxin substrates useful in assaying for the protease activity of any clostridial toxin, including botulinum toxins of all serotypes as well as tetanus toxins. A clostridial toxin substrate of the invention contains a donor fluorophore; an acceptor having an absorbance spectrum overlapping the emission spectrum of the donor fluorophore; and a clostridial toxin recognition sequence that includes a cleavage site, where the cleavage site intervenes between the donor fluorophore and the acceptor and where, under the appropriate conditions, resonance energy transfer is exhibited between the donor fluorophore and the acceptor.
Owner:ALLERGAN INC
Mutant botulinum neurotoxin serotype A polypeptide and uses thereof
Modified polypeptides based on the botulinum neurotoxin A heavy chain containing the double mutation Trp-Tyr->Leu-Ser in the ganglioside binding motif Ser-X-Trp-Tyr do not bind polysialogangliosides and nerve endings. The polypeptides are useful in the preparation of nontoxic vaccines against the effects of C. botulinum infection. The modified polypeptides are also useful as vehicles for the transepithelial delivery of diagnostic and therapeutic entities, through formation of conjugates between the polypeptides and the diagnostic or therapeutic entities.
Owner:THOMAS JEFFERSON UNIV
Recombinant light chains of botulinum neurotoxins and light chain fusion proteins for use in research and clinical therapy
Botulinum neurotoxins, the most potent of all toxins, induce lethal neuromuscular paralysis by inhibiting exocytosis at the neuromuscular junction. The light chains (LC) of these dichain neurotoxins are a new class of zinc-endopeptidases that specifically cleave the synaptosomal proteins, SNAP-25, VAMP, or syntaxin at discrete sites. The present invention relates to the construction, expression, purification, and use of synthetic or recombinant botulinum neutoroxin genes. For example, a synthetic gene for the LC of the botulinum neurotoxin serotype A (BoNT / A) was constructed and overexpressed in Escherichia coli. The gene product was purified from inclusion bodies. The methods of the invention can provide 1.1 g of the LC per liter of culture. The LC product was stable in solution at 4° C. for at least 6 months. This rBoNT / A LC was proteolytically active, specifically cleaving the Glu-Arg bond in a 17-residue synthetic peptide of SNAP-25, the reported cleavage site of BoNT / A. Its calculated catalytic efficiency kcat / Km was higher than that reported for the native BoNT / A dichain. Treating the rBoNT / A LC with mercuric compounds completely abolished its activity, most probably by modifying the cysteine-164 residue located in the vicinity of the active site. About 70% activity of the LC was restored by adding Zn2+ to a Zn2+-free, apo-LC preparation. The LC was nontoxic to mice and failed to elicit neutralizing epitope(s) when the animals were vaccinated with this protein. In addition, injecting rBoNT / A LC into sea urchin eggs inhibited exocytosis-dependent plasma membrane resealing.
Owner:UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE ARMY
Mutant botulinum neurotoxin serotype a polypeptide and uses thereof
Modified polypeptides based on the botulinum neurotoxin A heavy chain containing the double mutation Trp-Tyr->Leu-Ser in the ganglioside binding motif Ser-X-Trp-Tyr do not bind polysialogangliosides and nerve endings. The polypeptides are useful in the preparation of nontoxic vaccines against the effects of C. botulinum infection. The modified polypeptides are also useful as vehicles for the transepithelial delivery of diagnostic and therapeutic entities, through formation of conjugates between the polypeptides and the diagnostic or therapeutic entities.
Owner:THOMAS JEFFERSON UNIV
Small molecule inhibitors of botulinum neurotoxins
The invention provides potent quinolinol-based BoNT / A small-molecule inhibitors of botulinum neurotoxins, in particular of Clostridium botulinum serotype A neurotoxins. The invention also provides methods of using these small-molecule inhibitors to inhibit infections by Clostridium botulinum, as well as, methods of preventing infections by Clostridium botulinum through materials that may be ingested.
Owner:UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE ARMY
Fret protease assays for botulinum serotype a/e toxins
The present invention provides clostridial toxin substrates useful in assaying for the protease activity of any clostridial toxin, including botulinum toxins of all serotypes as well as tetanus toxins. A clostridial toxin substrate of the invention contains a donor fluorophore; an acceptor having an absorbance spectrum overlapping the emission spectrum of the donor fluorophore; and a clostridial toxin recognition sequence that includes a cleavage site, where the cleavage site intervenes between the donor fluorophore and the acceptor and where, under the appropriate conditions, resonance energy transfer is exhibited between the donor fluorophore and the acceptor.
Owner:ALLERGAN INC
Serum bactericidal assay for N. meningitidis specific antisera
The present invention relates to the field of Serum Bactericidal Activity (SBA) assays for Gram negative bacteria, in particular N. meningitidis. The SBA assay is the most important method for measuring functional activity of serum antibodies against meningococcus. In order to determine whether a subject or a population is seropositive against invasive meningococcus the SBA test should ideally be both sensitive and specific. The inventors have found the standard N. meningitidis serotype A and W SBAs can be significantly improved in this regard.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Rabbit hemorrhagic disease virus baculovirus vector and pasteurella multocida bivalent inactivated vaccine and preparation method thereof
ActiveCN108904796AFermentation culture process is matureImprove securityAntibacterial agentsSsRNA viruses positive-senseAdjuvantP. multocida
The invention relates to a rabbit hemorrhagic disease virus baculovirus vector and pasteurella multocida bivalent inactivated vaccine and a preparation method thereof, and belongs to the field of immune technology. Recombinant rabbit hemorrhagic disease virus VP60 baculovirus is inoculated into Sf9 insect cells and cultured at 27-28 DEG C. When cell lesion reaches 85% or more, a cell culture is harvested and inactivated, and the inactivated cell culture is used as a rabbit hemorrhagic disease virus antigen. Rabbit Pasteurella multocida capsular serotype A C51-17 strain is amplified and cultured, a bacterial solution is inactivated, and the inactivated bacterial solution is used as a Pasteurella multocida antigen. The rabbit hemorrhagic disease virus baculovirus vector and pasteurella multocida bivalent inactivated vaccine can be prepared by mixing the rabbit hemorrhagic disease virus antigen and the Pasteurella multocida antigen with adjuvants in proportion. The rabbit hemorrhagic disease virus baculovirus vector and pasteurella multocida bivalent inactivated vaccine has high safety, good immune effect and simple process, and can be used for preventing and controlling Rabbit Hemorrhagic Disease (Rabbit Plague) and Rabbit Pasteurella multocida.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
Bovine capsular serotype A Pasteurella mutocida, validation identification and application thereof
The invention discloses bovine capsular serotype A Pasteurella mutocida and application thereof. The bovine capsular serotype A Pasteurella mutocida is separated from disease samples with haemorrhagic septicomia of cattle in six provinces (cities) with the microbial preservation number of CGMCC No.3619. The bovine capsular serotype A Pasteurella mutocida is cultured by BHI, oil adjuvant is adopted to prepare inactivated immunogen, and a mouse model proves that the bovine capsular serotype A Pasteurella mutocida has complete protecting function on serotype A Pm velogenic attck, and does not have crossing protection with serotype B Pm. Testing results prove that vaccine can be prepared by the bovine capsular serotype A Pasteurella mutocida for preventing the haemorrhagic septicomia of cattle caused by the capsular serotype A Pasteurella mutocida. The strain cannot cause diseases when inoculated to chicks, which proves that the strain is chicken isolated capsular serotype A Pasteurella mutocida.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Application of botulinum neurotoxin serotype A in preparing medicine for treating raynaud syndrome
InactiveCN103705913ARelief or disappearance of symptoms and signsCertain curative effectNervous disorderPeptide/protein ingredientsSide effectBlood vessel
The invention relates to novel application of botulinum neurotoxin serotype A, namely novel application in preparing a medicine for treating raynaud syndrome. The botulinum neurotoxin serotype A is easy to use for operation, low in cost, safe and small in pain when used for treating raynaud syndrome, can overcome the defects that the medicine acts to blood vessels of a whole body in a general oral, intramuscular or intravenous administration, the dose is difficult to hold, the side effect is high and the like, and can also overcome the defects of large surgical wound, more complications and high relapse rate of surgical treatment, so that the application is an innovation to various traditional treatment means ideally and methodologically.
Owner:LIUZHOU WORKERS HOSPITAL +1
METHOD OF PRODUCING MENINGOCOCCAL MENINGITIS VACCINE FOR NEISSERIA MENINGITIDIS SERO TYPES A,C,Y, and W-135
InactiveUS20080020002A1Yield maximizationYield minimizationAntibacterial agentsBacteriaConjugate vaccineSynechococcus
Methods for producing quadrivalent meningococcal meningitis polysaccharide and conjugate vaccines for serotypes A, C, Y and W-135 disclosed. Neisseria meningitidis fastidious medium was designed to maximize the yield of capsular polysaccharides and generate minimal cellular biomass and endotoxin in a short duration of fermentation. The crude polysaccharides are isolated, purified, and mechanically depolymerized by sonication. These purified polysaccharides were found in human clinical trials to be safe and immunogenic against meningococcal disease caused by N. meningitidis A, C, Y and W-135 serogroups in sub-Saharan Africa. In the preferred embodiment, the polysaccharides are conjugated to carrier proteins of diphtheria or tetanus toxiod to an average molecular size of 5100 to 9900 Daltons and provide broad spectrum protection to humans of all ages. Accelerated polysaccharide production and the efficacy of the resulting vaccine are demonstrated.
Owner:REDDY JEERI R
Antibody against serotype a lipopolysaccharide of pseudomonas aeruginosa
InactiveUS20130004500A1High antibacterial activityEffective treatmentAntibacterial agentsAnimal cellsPulmonary infectionAntibacterial activity
Provided is a novel antibody having an excellent antibacterial activity against P. aeruginosa. By using plasmablasts obtained from cystic fibrosis patients with chronic P. aeruginosa pulmonary infection as starting materials, antibodies which bind to LPS of a P. aeruginosa strain of serotype A and which have excellent antibacterial activities in vitro and in vivo were successfully obtained.
Owner:MEIJI SEIKA PHARMA CO LTD +1
Novel recombinant botulinum neurotoxins with increased duration of effect
ActiveUS20200131494A1Extended durationHigh specific biological activityPeptide/protein ingredientsPeptidesDrug biological activityStereochemistry
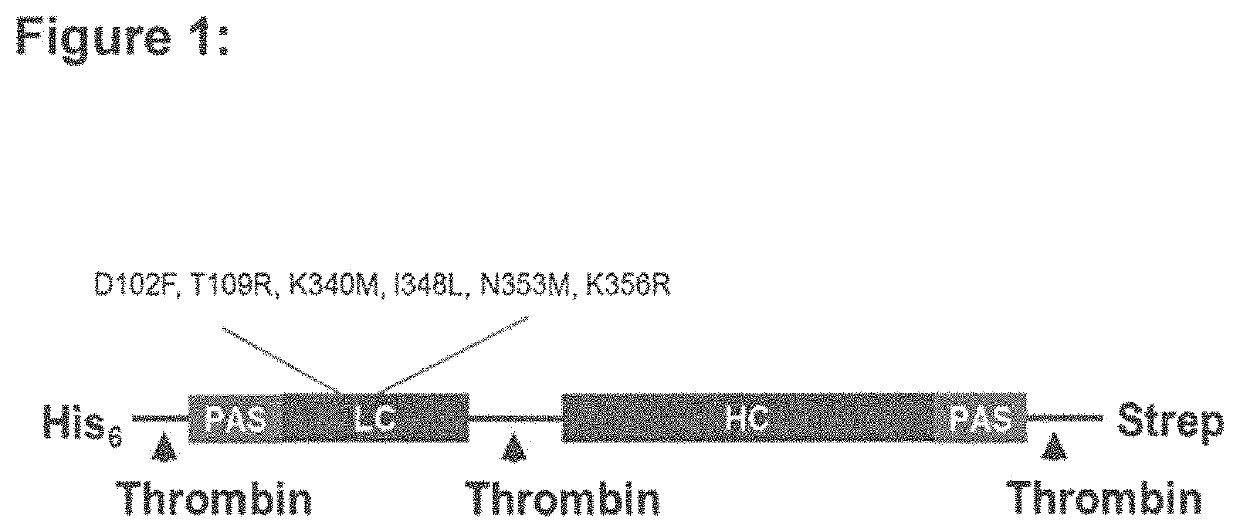
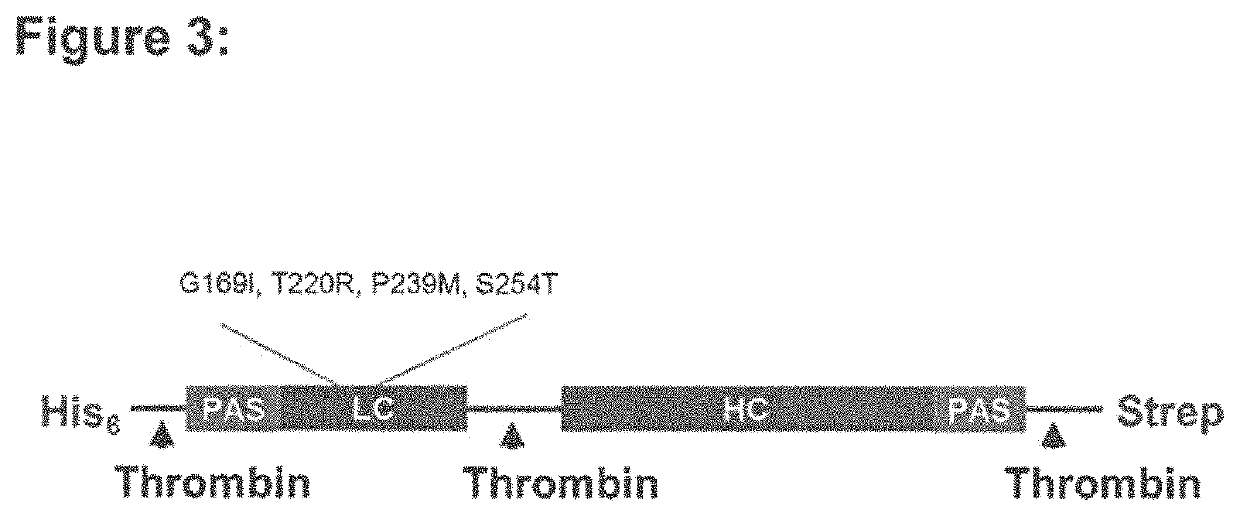
This invention relates to novel recombinant botulinum neurotoxins serotype A exhibiting both (i) an increased duration of effect and (ii) a high specific biological activity. These novel recombinant botulinum neurotoxins comprise at least two additional domains consisting of proline, alanine and an additional amino acid residue and at least one amino acid modification which is located at the alpha-exosite or at the beta-exosite of the light chain of the neurotoxin. The invention further relates to novel recombinant single-chain precursor botulinum neurotoxins and compositions comprising the recombinant botulinum neurotoxin with an increased duration of effect and a high specific biological activity.
Owner:MERZ PHARMA GMBH & CO KGAA
Serum Bactericidal Assay for N. Meningitidis Specific Antisera
The present invention relates to the field of Serum Bactericidal Activity (SBA) assays for Gram negative bacteria, in particular N. meningitidis. The SBA assay is the most important method for measuring functional activity of serum antibodies against meningococcus. In order to determine whether a subject or a population is seropositive against invasive meningococcus the SBA test should ideally be both sensitive and specific. The inventors have found the standard N. meningitidis serotype A and W SBAs can be significantly improved in this regard.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Enhanced substrates for the protease activity of serotype a botulinum neurotoxin
Substrates of botulinum toxin serotype A (BoNT A), kits comprising the substrates, and methods of using the substrates are disclosed. BoNT A cleaves SNAP-25 and the substrates that are based upon a portion of SNAP-25. Various amino acid modifications to the sequence of the peptide based on SNAP-25 are performed. Fluorescence labels that act as donors and acceptors may be added to the substrate to aid in the study of BoNT A.
Owner:SCHMIDT JAMES J +1
Vaccine Protection Assay
InactiveUS20080064057A1Minimize impactAnimal cellsMicrobiological testing/measurementSerum igeFunctional activity
The present invention relates to the field of Serum Bactericidal Activity (SBA) assays for Gram negative bacteria, in particular N. meningitidis. The SBA assay is the most important method for measuring functional activity of serum antibodies against meningococcus. In order to determine whether a subject or a population is seropositive against invasive meningococcus the SBA test should ideally be both sensitive and specific. The inventors have found the standard N. meningitidis serotype A and W SBAs can be significantly improved in this regard.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Novel recombinant botulinum neurotoxins with increased duration of effect
PendingUS20210008156A1Increased duration of effectThe method is accurate and reliablePeptide/protein ingredientsPeptidesHeavy chainViral protease
The invention relates to novel recombinant single-chain precursor botulinum neurotoxins serotype A comprising at least one additional domain and least one amino acid modification of the heavy chain of the neurotoxin. The novel recombinant single-chain precursor botulinum neurotoxins further comprises at least one cleavage site for a protease selected from the group consisting of thrombin, HRV3C, Tobacco Etch Vims protease, enterokinase and factor Xa. The invention further relates to novel recombinant botulinum neurotoxins serotype A exhibiting an increased duration of effect.
Owner:MERZ PHARMA GMBH & CO KGAA
Plasmid-encoded neurotoxin genes in Clostridium botulinum serotype A subtypes
ActiveUS8435759B2Increasing therapeutic applicationMethod can be usedPeptidesFermentationNeurexinBiology
The present invention provides a novel isolated plasmid, wherein the plasmid is a native plasmid found in unique C. botulinum type A strains and encode either BoNT / A3 or BoNT / A4 and BoNT / B. The present invention also provides a method of obtaining a plasmid-encoded botulinum neurotoxin and botulinum neurotoxin complex comprising the step of isolating a plasmid encoding the cntA / A or cntA / B neurotoxin gene and genes encoding protein components of the toxin complex from a C. botulinum type A strain. The inventors performed comparative analyzes of representative BoNT / A subtype strains by pulsed-field gel electrophoresis (PFGE) and Southern hybridizations with probes specific for the BoNT / A and B genes, cntA / A and cntA / B. Unexpectedly, the inventors determined that the genes encoding BoNT / A3 in the A3 strain, and BoNT / A4 and BoNT / B in the A4 strain, are on plasmids.
Owner:WISCONSIN ALUMNI RES FOUND
Method and kit for detecting antibody to avibacterium paragallinarum
ActiveUS20110201034A1High detection sensitivityIncrease concentrationPeptide/protein ingredientsPeptide preparation methodsHaemophilusELISA unit
A method and a kit for detecting an antibody to Avibacterium paragallinarum are provided. A method for detecting an antibody to Avibacterium paragallinarum which comprises detecting an antibody induced by an outer-membrane protein of Avibacterium paragallinarum serotype A and / or serotype C by ELISA with a solid phase to which a peptide consisting of an amino acid sequence of non-homologous region of said outer-membrane protein or a portion thereof is immobilized, and a detection kit used for said method.
Owner:KM BIOLOGICS CO LTD
Anti-Mycoplasma bovis and Pasteurella fusion protein md‑UF1‑Md‑AP2
The invention discloses an anti-Mycoplasma bovis and Pasteurella fusion protein Md-UF1-Md-AP2 and a gene encoding the fusion protein. It induces housefly larvae with Mycoplasma pneumoniae and constructs an inhibitory subtractive library. In the inhibitory subtractive library The Md-UF1 gene screened out in the chicken-derived Salmonella housefly larvae was used to construct a suppressive subtractive library, and the Md-AP2 gene screened out in the suppressive subtractive library was constructed using the overlap extension and shearing technique. The fusion gene Md-UF1-Md-AP2, the expressed anti-Mycoplasma bovis and Pasteurella fusion protein Md-UF1-Md-AP2, has inhibitory effects on Pasteurella multocida type A and Mycoplasma bovis, The problem of drug resistance of Pasteurella bovis and Mycoplasma bovis has been solved. When the cattle have pneumonia symptoms, early medication can prevent and treat at the same time.
Owner:JILIN AGRICULTURAL UNIV
Plasmid-encoded neurotoxin genes in clostridium botulinum serotype a subtypes
ActiveUS20100311118A1Increasing therapeutic applicationMethod can be usedPeptidesFermentationSerotypePlasmid
The present invention provides a novel isolated plasmid, wherein the plasmid is a native plasmid found in unique C. botulinum type A strains and encode either BoNT / A3 or BoNT / A4 and BoNT / B. The present invention also provides a method of obtaining a plasmid-encoded botulinum neurotoxin and botulinum neurotoxin complex comprising the step of isolating a plasmid encoding the cntA / A or cntA / B neurotoxin gene and genes encoding protein components of the toxin complex from a C. botulinum type A strain. The inventors performed comparative analyses of representative BoNT / A subtype strains by pulsed-field gel electrophoresis (PFGE) and Southern hybridizations with probes specific for the BoNT / A and B genes, cntA / A and cntA / B. Unexpectedly, the inventors determined that the genes encoding BoNT / A3 in the A3 strain, and BoNT / A4 and BoNT / B in the A4 strain, are on plasmids.
Owner:WISCONSIN ALUMNI RES FOUND
Vaccines and methods for creating a vaccine for inducing immunity to all dengue virus serotypes
ActiveUS10675343B2SsRNA viruses positive-senseViral antigen ingredientsNeutralising antibodyChimera Protein
A method to produce a chimeric protein having a flavivirus backbone and portions dengue virus is provided. The flavivirus envelope protein, such as from yellow fever virus 17D vaccine strain, is modified replacing amino acids surrounding the fusion loop of the flavivirus backbone with corresponding amino acids from the dengue virus envelope protein. The chimeric protein is useful as a vaccine to stimulate an immune response against DENV infection, thereby producing broadly neutralizing (protective) antibodies against dengue virus and reduce the induction of non-neutralizing antibodies that will cause enhancement.
Owner:MICHAEL SCOTT F +1
Antibovine mycoplasma and Pasteurella multocida mixed protein spray
InactiveCN104771755AHas inhibitory effectGood effectAntibacterial agentsGenetic material ingredientsBovine respiratory diseaseDisease
The invention discloses an antibovine mycoplasma and Pasteurella multocida mixed protein spray. Every 100g of the matrix of the spray contains 3-5g of an antibovine mycoplasma-containing protein Md-UF1 and 3-5g of an antibovine Pasteurella multocida protein Md-AP2, and the matrix is a gel matrix. Gene Md-UF1 screened from a suppression subtractive library constructed after inducing housefly larvae by a pathogen through the inventor and expressing an antibovine mycoplasma protein and gene Md-AP2 screened from the library and expressing antibovine capsular serotype A protein are used to construct fusion gene, the fusion gene expresses proteins, and experiment results prove that the spray has an inhibition effect on bovine mycoplasma and bovine Pasteurella multocida. The spray has good effects in the clinical treatment of bovine respiratory diseases induced by bovine mycoplasma and Pasteurella multocida mixed infection. The spray can be used in the early stage of the bovine respiratory disease.
Owner:JILIN AGRICULTURAL UNIV
A bivalent inactivated vaccine of avian nasal and tracheal avian bacillus a/b serotype
ActiveCN107550863BPrevent Respiratory DiseasesAntibacterial agentsBacterial antigen ingredientsBeta-glucanRespiratory disease
The invention relates to the field of immunology, and particularly discloses a poultry ornithobacterium rhinotracheale vaccine. The vaccine is prepared by taking isolated strains of two main epidemicserotypes of ornithobacterium rhinotracheale as antigens, culturing with a blood bouillon culture-medium, then inactivating by formaldehyde, mixing the antigens of the two serotypes at a ratio of 1 to1, then adding poloxamer with volume being 1 to 5 percent of that of the liquid antigens and beta glucan with volume being 1 to 4 percent of that of the liquid antigens to prepare a water phase, finally emusifying according to a ratio of the water phase to an oil phase being 4 to 6. The vaccine provided by the invention can be used for preventing symptoms such as respiratory diseases caused by currently epidemic poultry ornithobacterium rhinotracheale, slow growth and egg-laying decrease.
Owner:何诚 +3
Recombinant botulinum neurotoxins with increased duration of effect
ActiveUS11155802B2Increase their duration of effectExtended durationPeptide/protein ingredientsPeptidesDrug biological activityBiological organism
Owner:MERZ PHARMA GMBH & CO KGAA
Methods for detecting c. canimorsus capsular serotypes in a sample
Described herein are methods for identifying C. canimorsus in a sample as one of serotype A, serotype B, serotype C, serotype D or serotype E, amplification primer pairs, sets of primer pairs, oligonucleotide probes, sets of oligonucleotide probes, polyclonal antibodies, sets of polyclonal antibodies and kits that can be used for such methods. The application further provides polyvalent vaccines for the protection against an infection with C. canimorsus, methods of preparing said polyvalent vaccines and uses of these polyvalent vaccines.
Owner:UNIV DE NAMUR ASBL
Vaccines and methods for creating a vaccine for inducing immunity to all dengue virus serotypes
ActiveUS20200129607A1High affinityBroad but intermediate neutralization activitySsRNA viruses positive-senseViral antigen ingredientsNeutralising antibodyImmunity
A method to produce a chimeric protein having a Flavivirus backbone and portions dengue virus is provided. The Flavivirus envelope protein, such as from yellow fever virus 17D vaccine strain, is modified replacing amino acids surrounding the fusion loop of the Flavivirus backbone with corresponding amino acids from the dengue virus envelope protein. The chimeric protein is useful as a vaccine to stimulate an immune response against DENV infection, thereby producing broadly neutralizing (protective) antibodies against dengue virus and reduce the induction of non-neutralizing antibodies that will cause enhancement.
Owner:MICHAEL SCOTT F +1
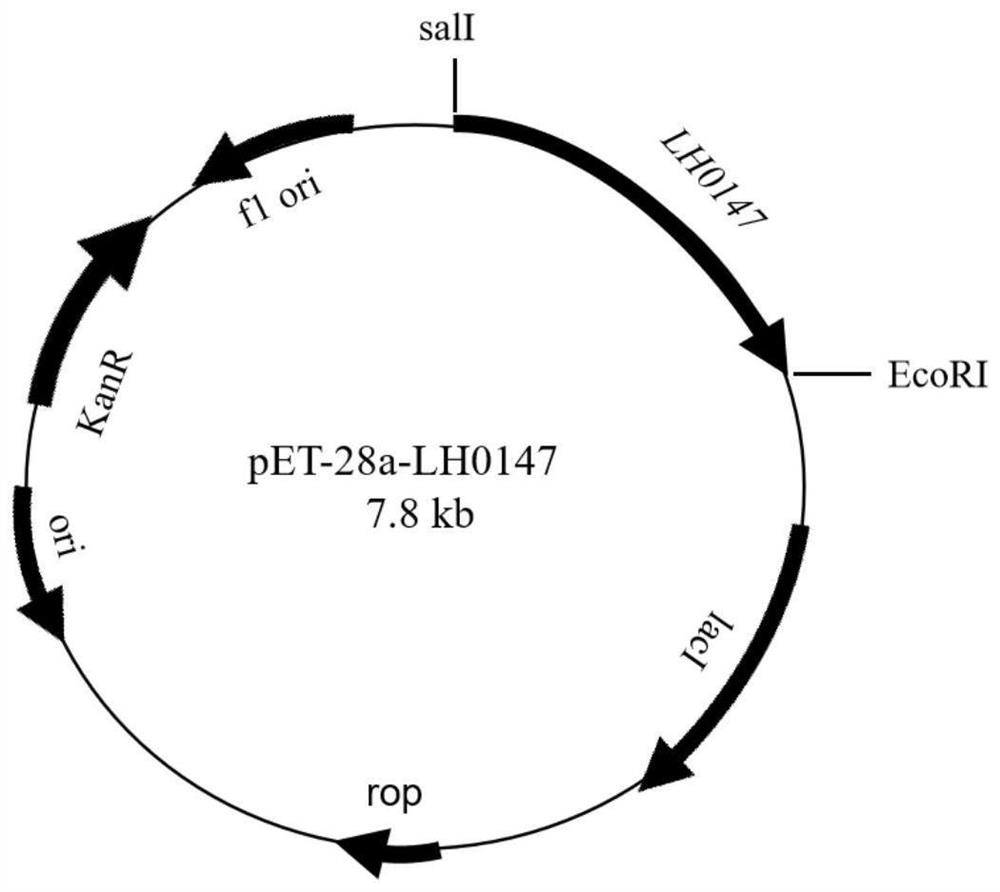
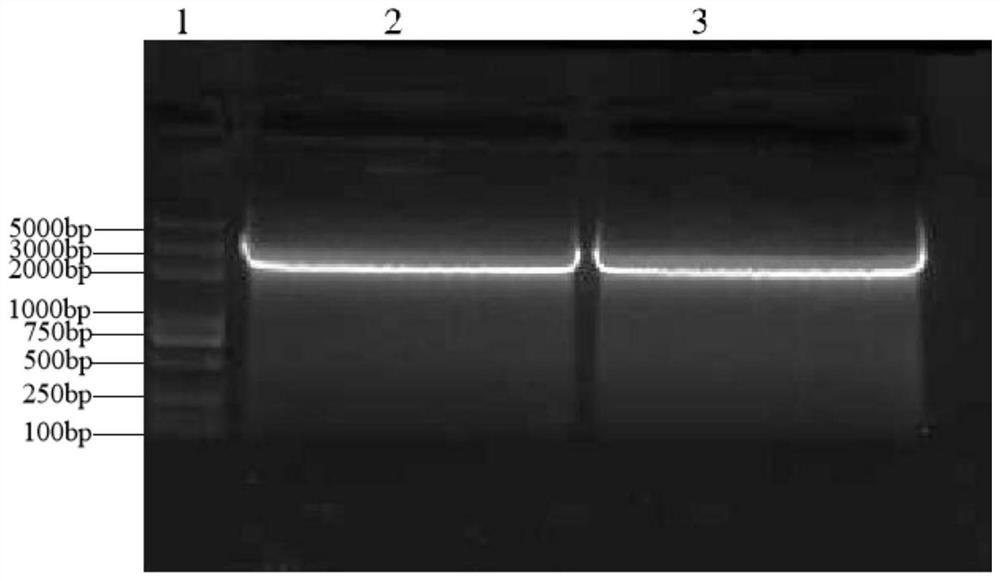
LH0147 gene, and expression vector, expression product and application thereof
InactiveCN112010951AHelp with researchAids in the study of pathogenic mechanismsBacteriaMicroorganism based processesP. multocidaEngineered genetic
The invention discloses a bovine-derived serotype A pasteurella multocida adhesin LH0147 gene as well as an expression vector, an expression product and application of the bovine-derived pasteurella multocida adhesin LH0147 gene. The LH0147 gene is shown as SEQ ID NO.1; the amino acid sequence of the LH0147 protein encoded by the LH0147 gene is shown as SEQ ID NO. 2; the recombinant expression vector of the LH0147 gene can be used for expressing the LH0147 protein in vitro; the specific method comprises the following steps: performing cloning to obtain the LH0147 gene according to claim 1, andinserting the LH0147 gene between an enzyme cutting site salI and an EcoRI of an expression plasmid pET-28a to obtain a recombinant expression vector pET-28a-LH0147; converting the recombinant expression vector ET-28a-LH0147 into a competent cell BL21 (DE3) through heat shock, and performing screening to obtain a recombinant genetically engineered bacterium; inoculating the recombinant genetically engineered bacterium in vitro, and inducing the recombinant genetically engineered bacterium to express the LH0147 protein. According to the invention, the gene sequence of the bovine-derived serotype A pasteurella multocida adhesin LH0147 is expressed in vitro for the first time, so that the function and pathogenesis of the protein can be researched.
Owner:NANJING AGRICULTURAL UNIVERSITY
Botulinum neurotoxin-specific capture agents, compositions, and methods of using and making
ActiveCN107624115APolypeptide with localisation/targeting motifAntibody mimetics/scaffoldsDiseaseClick chemistry
The application provides stable peptide-based Botulinum neurotoxin (BoNT) serotype A capture agents and methods of use as detection and diagnosis agents and in the treatment of diseases and disorders.The application further provides methods of manufacturing BoNT serotype A capture agents using iterative on-bead in situ click chemistry.
Owner:CALIFORNIA INST OF TECH +1
Recombinant avian infectious coryza vaccine and process for preparing same
InactiveUS20120003257A1Improve purification effectLow production costAntibacterial agentsBacterial antigen ingredientsInclusion bodiesTGE VACCINE
A recombinant avian infectious coryza vaccine and a process for preparing the same are provided. A process for preparing a recombinant avian infectious coryza vaccine which comprises step of constructing E. coli that may produce as an inclusion body a fusion peptide consisting of peptides derived from outer-membrane protein of Avibacterium paragarinarum serotype A and serotype C, step of culturing said E. coli and colleting and purifying inclusion body from culture, and step of preparing a preparation comprising said purified inclusion body, and an avian infectious coryza vaccine comprising as an active ingredient the fusion peptide. A linker sequence may be inserted between the respective peptides comprising the fusion peptide. For the peptide derived from the serotypes A and C, an amino acid sequence region of Region 2 or its vicinity responsible for protection from infection may be used.
Owner:KM BIOLOGICS CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com