A bivalent inactivated vaccine of avian nasal and tracheal avian bacillus a/b serotype
A bivalent inactivated vaccine and serotype technology, which is applied in the direction of antibacterial drugs, bacterial antigen components, emulsion delivery, etc., can solve the problem of unclear relationship between pathogenicity and serotype
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] The identification of embodiment 1 nasal trachea ornithosis and the preparation of inactivated vaccine
[0035] 1. Materials
[0036] Seed production strains: ORT-SD (type B), ORT-98 (type A) were isolated and identified from our research group;
[0037]Attacking strain: ORT-98, serotype A, donated by researcher Chen Xiaoling of Beijing Academy of Agriculture and Forestry;
[0038] Adjuvant: No. 10 light white oil: purchased from Hangzhou Refinery of Sinopec Group;
[0039] Water-soluble β-1,3 / 1,6 glucan: purchased from Angel Yeast Co., Ltd., with a content of 90%.
[0040] Poloxamer 407: purchased from BASF (China) Co., Ltd.
[0041] 2. Method
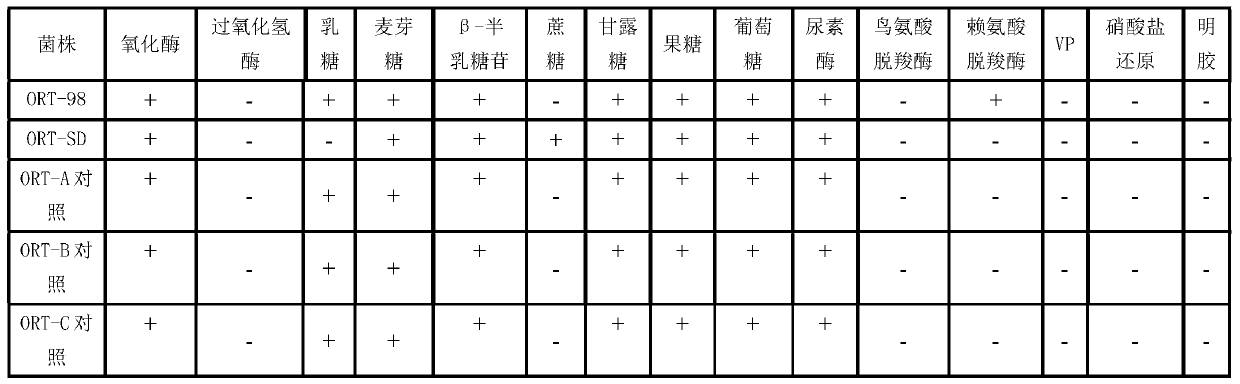
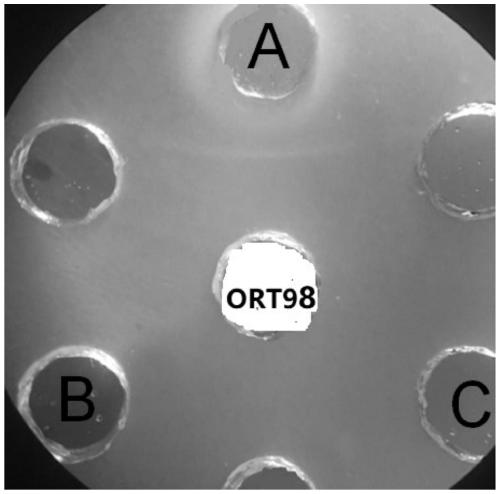
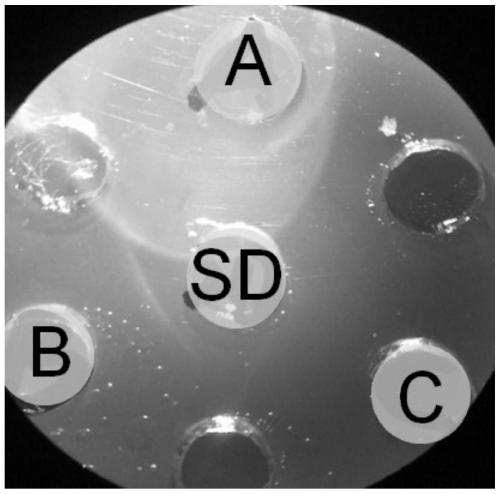
[0042] Vaccine antigen preparation: the two strains of avian nasal trachea aviophilum seed bacteria ORT-SD and ORT-98 strains of the present invention are first biochemically identified, and the results are as follows: figure 1 As shown, the ORT isolates were positive for glycosides such as glucose, lactose, fructose, and ...
Embodiment 2
[0078] Example 2 Screening of nasal and tracheal avian bacilli immune adjuvants
[0079] This example aims to use white oil and / or β-1,3 / 1,6 glucan as an immune adjuvant to emulsify with nasal and tracheal A. immune efficacy.
[0080] 1. Materials
[0081] 1.1 Strains and experimental animals
[0082] Seed production strains: ORT-SD (type B), ORT-98 (type A) were isolated and identified from our research group;
[0083] Attacking strain: ORT-98, serotype A, donated by researcher Chen Xiaoling of Beijing Academy of Agriculture and Forestry;
[0084] Experimental animals: 70 3-week-old SPF chickens, purchased from Beijing Weitong Meria Experimental Animal Co., Ltd.
[0085] 1.2 Main reagents and kits
[0086] Chicken cytokine ELISA antibody detection kit: purchased from U.S. Kingfisher Inc;
[0087] ORT antibody detection ELISA kit, purchased from American IDEXX company;
[0088] Control group vaccine Nobilis OR Inac: purchased from Intervet Company in the Netherlands, ma...
Embodiment 3
[0134] Example 3 The immune protection test of nasal trachea ornithosis to ducks
[0135] This example aims to evaluate the immune efficacy of the ORT bivalent inactivated oil emulsion vaccine prepared with white oil + dextran as an adjuvant on ducks.
[0136] 1. Materials
[0137] 1.1 Reagents and media:
[0138] 5% sheep blood agar plate or chocolate agar plate and blood agar medium: purchased from Beijing Guangda Hengyi Technology Co., Ltd.;
[0139] Control group vaccine: purchased from Israel ABIC Company, mainly containing ORT of three serotypes A, B, and C.
[0140] 1.2 Experimental animals: 40 1-day-old healthy Peking ducks, the breed of Beijing Jinxing ducks, were tested negative for avian bacilli in the nose and trachea, and were purchased from the duck farm of Nankou Farm, Beijing.
[0141] 1.3 strains:
[0142] Attacking strain: ORT-98, serotype A, donated by researcher Chen Xiaoling of Beijing Academy of Agriculture and Forestry;
[0143] Attacking strain: OR...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com