Rabbit hemorrhagic disease virus baculovirus vector and pasteurella multocida bivalent inactivated vaccine and preparation method thereof
A technology of rabbit hemorrhagic virus and baculovirus vector, applied in the field of immunization, can solve the problems of large individual differences in animals, the preparation process of Pasteurella multocida is not stable enough, the spread of virulent and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
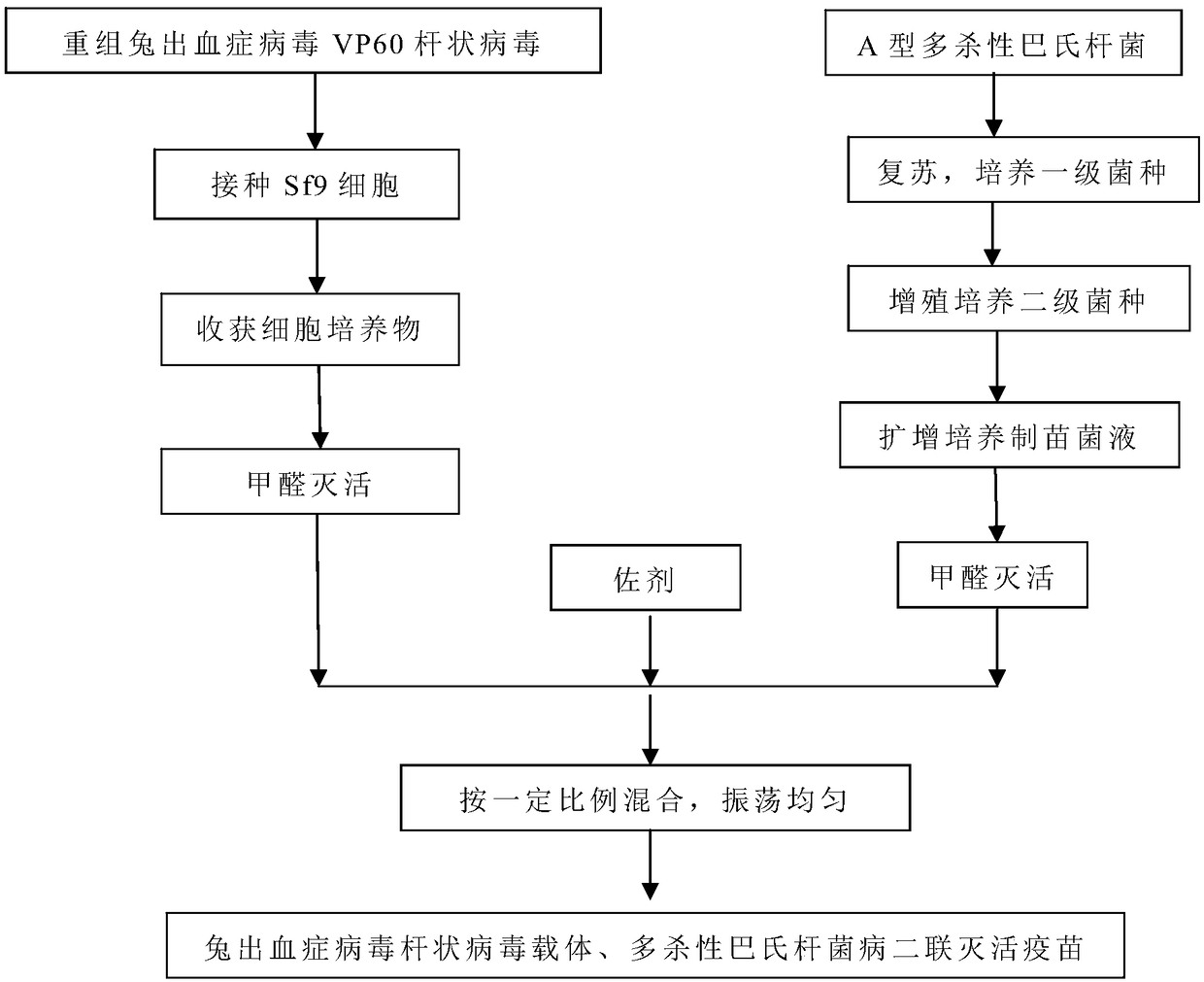
[0070] Embodiment 1 recombinant rabbit hemorrhagic disease virus VP60 protein antigen cell culture tank suspension culture method
[0071] Cultivate the Sf9 cells in suspension culture at 27-28°C and 90-120r / min for 24-48 hours, continuously passage and expand, the passage ratio is 1:2-1:4, inoculate the cell culture tank, and first reach the culture tank In the process of cultivating cells in the cell culture tank, control the parameters of the cell culture tank within the normal range: temperature 27-28°C, pH value 6.0-6.2, dissolved oxygen (DO) 50% ~ 60%, stirring speed 50 ~ 70 r / min. After the cells in the cell culture tank reached a certain density (about 2×10 6 Individual / ml), inoculate the recombinant baculovirus at a ratio of 1% to carry out virus maintenance culture, and control the parameters such as temperature, pH value, DO, stirring speed, etc. in a suitable range. 4 to 5 days after inoculation, when the diseased cells reach more than 85%, the cell culture can b...
Embodiment 2
[0074] Example 2 Preparation of Rabbit Hemorrhagic Disease Virus Baculovirus Vector Cell Culture Inactivation Solution
[0075] Add formaldehyde solution with a final concentration of 0.2% to the recombinant rabbit hemorrhagic disease virus VP60 baculovirus cell culture prepared above, mix well, change the tank and inactivate at 37°C for 18-24 hours, stirring at a speed of 50-70r / min or inactivate at 37°C for 24 hours after changing the bottle, shake once every 2 to 3 hours for 3 minutes each time to obtain the inactivation solution of rabbit hemorrhagic virus baculovirus vector cell culture, and place the inactivation solution in 2 to 3 hours. Store at 8°C for no more than 6 months. In the inactivation test, take the inactivation solution of the rabbit hemorrhagic disease virus baculovirus carrier cell culture, inoculate well-grown Sf9 cells according to 1% of the total amount, culture at 27-28°C, and observe continuously for 5 days. There is no cytopathic change. Harvest th...
Embodiment 3
[0076] Embodiment 3 Pasteurella multocida fermentation culture method
[0077] 3 Preparation of Pasteurella multocida fermentation broth
[0078]3.1 Propagation and identification of primary seeds Dilute the freeze-dried bacteria of Pasteurella multocida C51-17 strain with sterilized physiological saline, and then streak and inoculate them on a Martin agar plate containing 4% serum and 0.1% lysed whole blood. Incubate at 37°C for 18-22 hours, select at least 5 typical colonies, inoculate several branches of Martin agar slant containing about 4% serum and 0.1% lysed whole blood, and culture at 37°C for 18-22 hours, as a primary seed. Store at 2-8°C and use for no more than 7 days.
[0079] 3.2 Propagation and identification of secondary seeds Take primary seeds and inoculate them in Martin broth containing 0.5% serum, place at 37°C, 200r / min, vibrate and cultivate for 14-18 hours, take samples for pure inspection, and use them as secondary seeds after passing the test. Store...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com