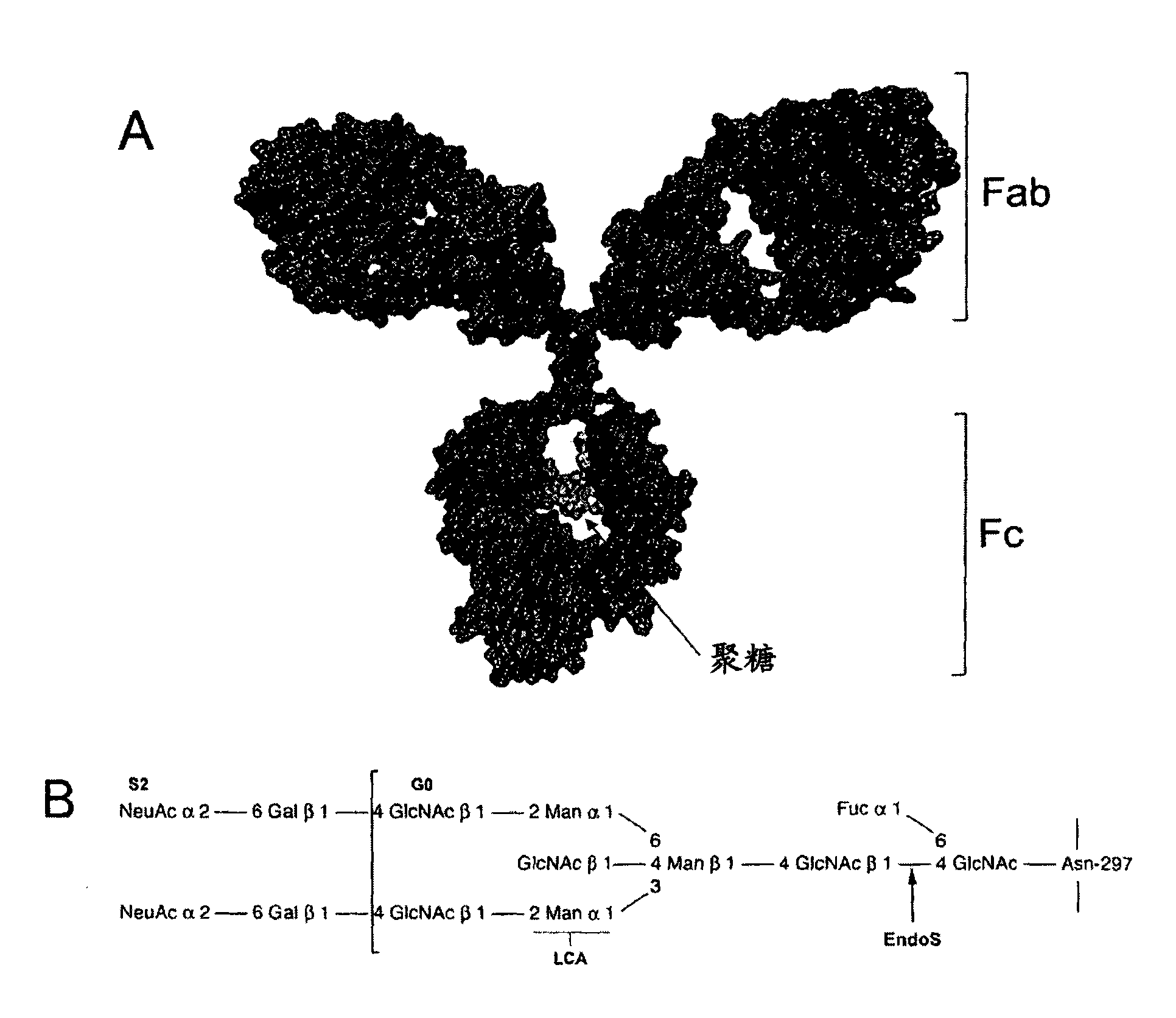Use of the endoglycosidase endos for treating immunoglobulin g mediated diseases
An endoglycosidase, application technology, applied in endocrine system diseases, sexual diseases, metabolic diseases and other directions, can solve problems such as undiscovered
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0114] Example 1: EndoS efficiently hydrolyzes IgG in human blood
[0115] To be effective as a therapeutic against pathological IgG, EndoS needs to be active at low concentrations in the context of human whole blood. To investigate this, recombinant EndoS (rEndoS) without a signal sequence (ie, having the sequence of SEQ ID NO: 1 ) was produced and purified as previously described (Collin & Olsén, 2001, Infect. Immun. 69:7187-7189). Increasing final concentrations (0, 0.31, 0.63, 1.25, 2.5, 5, 10 and 20 μg / ml) of rEndoS were rolled end over end in 500 μl of heparinized human blood from healthy volunteers at 37°C Incubate with rotation for 1 hour. Samples were centrifuged at 720 xg for 10 min at 4°C, after which IgG in plasma was purified using protein G sepharose according to the manufacturer's instructions (GE Healthcare Bjosciences, Uppsala, Sweden). Consistent with previous findings (Collin & Olsén 2001, EMBO J. 20:3046-3055) on the IgG binding proteins Protein H (from...
Embodiment 2
[0118] Example 2: EndoS efficiently hydrolyzes IgG in rabbits
[0119] To further substantiate the utility of EndoS as a therapeutic agent, the IgG glycan hydrolytic activity of EndoS in the circulation of living animals was studied. Swedish loop rabbits weighing approximately 3 kg were injected intravenously with 1 mg of rEndoS, corresponding to an rEndoS:IgG ratio of approximately 1:2000 (assuming that rEndoS is only distributed in the blood). Animals showed no signs of disease. Serum samples were collected at 0, 1, 2, 4, 6, 8 and 12 hours and on days 1, 2, 3, 4, 5, 6, 8 and 10. Serum IgG was analyzed for glycosylation status by SDS-PAGE and lectin blot analysis as described previously for human blood.
[0120] These experiments showed that, prior to injection, the apparent molecular weight of the IgG heavy chain was comparable to that of fully glycosylated intact rabbit IgG ( Image 6 A, staining, 0 hours and IgG). In contrast, there was already a partial shift of the...
Embodiment 3
[0124] Example 3: EndoS is active in rabbits despite antibodies against the EndoS enzyme
[0125] Since EndoS was fully active at the second and third injections, it was intriguing to determine whether this was due to an absent or low level of immune response to the enzyme or to have specific antibodies against EndoS that did not interfere with enzymatic activity. Interested in. Since it is known that healthy individuals and those infected with S. pyogenes have antibodies against EndoS ( This is of particular interest since et al., 2004, J. Infect. Dis. 189:797-804).
[0126] To investigate this, purified rEndoS was separated on 10% SDS-PAGE and electroblotted onto PVDF cut into 1.5 mn strips. Strips were incubated with a 1:500 dilution of all serum samples from the first, second and third injections, followed by incubation with peroxidase-labeled goat anti-rabbit antibody (Pierce). Strips were developed with chemiluminescence as described above for lectin blots.
[012...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com