Product for malignant tumor related screening and assessing, and application and method thereof
A technology for malignant tumors and products, applied in the fields of biotechnology and medicine, it can solve the problems of difficult comprehensive consideration of detection, low sensitivity and specificity, and a positive rate of less than 70%.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0322] Example 1.Gal ratio is used for the screening / screening of malignant tumors
[0323] The degree of IgG galactosylation in control serum samples (n=924) and malignant tumor patient serum samples (n=1737) was determined using the materials and methods described above. The test results are as follows:
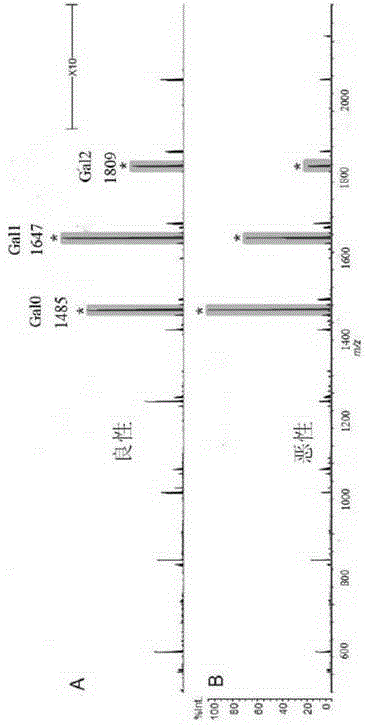
[0324] 1. By observing the MS spectra of a total of 2661 serum IgGN-sugar chains, it can be found that there are significant differences in the Gal ratio between malignant tumor patients and controls. Representative spectra such as figure 1 shown.
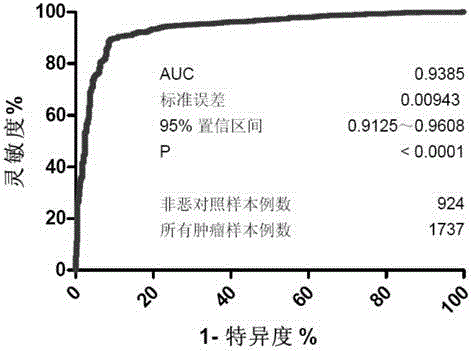
[0325] 2. The Gal ratio of 2661 samples was compared, and the receiver operating characteristic curve (receiver operating characteristic curve, ROC curve) analysis was carried out at the same time. The results show that: using the Gal ratio as the tumor screening index, the AUC is 0.9385 (95% confidence interval: 0.9125-0.9608), and the corresponding specificity is as high as 88% when the control sensitivity is 90% (see F...
Embodiment 2
[0342] Example 2. Gal ratio for distinguishing ovarian cancer patients from controls (healthy and benign disease)
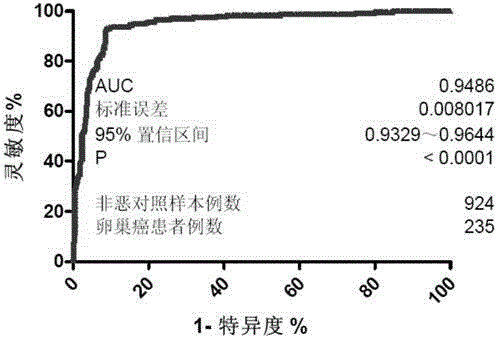
[0343] Samples obtained from ovarian cancer patients (n=235) and controls (n=924) obtained AUC=0.9486 (95% confidence interval: 0.9329, 0.9644), see Figure 2B .
[0344] The mean Gal ratio of ovarian cancer patients was significantly different from that of control samples (t test, p<0.0001). (See Table 3A).
[0345] The above analysis confirmed that the serum IgG galactosylation index of the present invention can effectively screen and distinguish ovarian cancer patients and controls with high sensitivity and specificity.
Embodiment 3
[0346] Example 3. Gal Ratio Used to Distinguish Lung Cancer Patients from Controls
[0347] AUC=0.9578 (95% confidence interval: 0.9433, 0.9724) obtained from samples obtained from lung cancer patients (n=206) and controls (healthy persons and benign diseases) (n=924), see Figure 2C .
[0348] The mean Gal ratio of lung cancer patients was significantly different from that of control samples (t test, p<0.0001) (see Table 3A).
[0349] The above analysis proves that the serum IgG galactosylation index of the present invention can effectively screen and distinguish lung cancer patients from controls with high sensitivity and specificity.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com