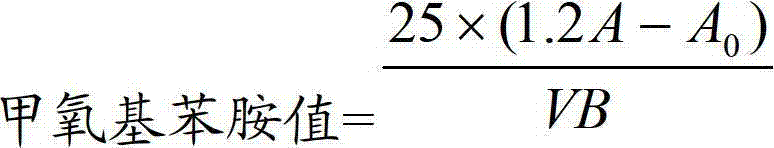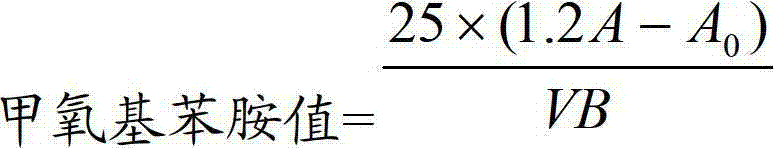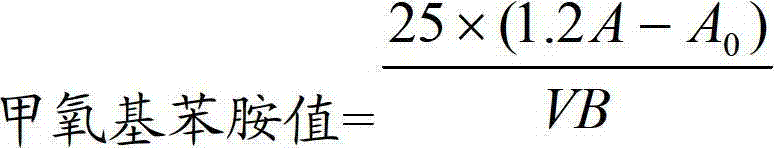Method for determining anisidine value of lipid emulsion
A technology of methoxyaniline value and fat emulsion, which is applied in the preparation of test samples and the measurement of color/spectral properties, etc., can solve the problems of inaccurate measurement results and inability to measure methoxyaniline.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Precisely measure 10ml of propofol injection and place it in a separatory funnel.
[0048] Add 1ml of 2mol / L hydrochloric acid, add 1ml of ethanol, 10ml of dichloromethane or chloroform along the bottle wall, and shake vigorously.
[0049] Static samples cannot be separated, so dichloromethane and chloroform are not suitable as extraction agents.
Embodiment 2
[0051] Precisely measure 10ml of propofol injection and place it in a separatory funnel.
[0052] Add 1ml of 2mol / L hydrochloric acid, add 1ml of ethanol and 10ml of ethyl acetate along the bottle wall, and shake vigorously.
[0053] The layers were left to stand, and the upper oil phase was transferred to a 250ml flask. The lower aqueous phase was extracted twice with ethyl acetate, each time with 10 ml of ethyl acetate, and the oil phases were combined, namely ethyl acetate liquid.
[0054] Concentrate below 40°C with a rotary evaporator.
[0055] The residue was dissolved in isooctane and transferred to a 25ml measuring bottle and diluted to the mark with isooctane as the test solution.
[0056] Take the test solution, take isooctane as a blank, and measure the absorbance value at 350nm according to UV-Vis spectrophotometry, and the absorbance value is A0.
[0057]Accurately measure 10ml of the test solution, put it into a brown stoppered test tube, accurately add 2ml of...
Embodiment 3
[0065] Precisely measure 10ml of propofol injection and place it in a separatory funnel.
[0066] Add 1ml of 2mol / L hydrochloric acid, add 1ml of ethanol and 10ml of ethyl acetate along the bottle wall, and shake vigorously.
[0067] The layers were left to stand, and the upper oil phase was transferred to a 250ml flask. The lower aqueous phase was extracted twice with ethyl acetate, each time with 10 ml of ethyl acetate, and the oil phases were combined, namely ethyl acetate liquid.
[0068] Concentrate below 60°C with a rotary evaporator.
[0069] The residue was dissolved in isooctane and transferred to a 25ml measuring bottle and diluted to the mark with isooctane as the test solution.
[0070] Take the test solution, take isooctane as a blank, and measure the absorbance value at 350nm according to UV-Vis spectrophotometry, and the absorbance value is A0.
[0071] Accurately measure 10ml of the test solution, put it into a brown stoppered test tube, accurately add 2ml o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com