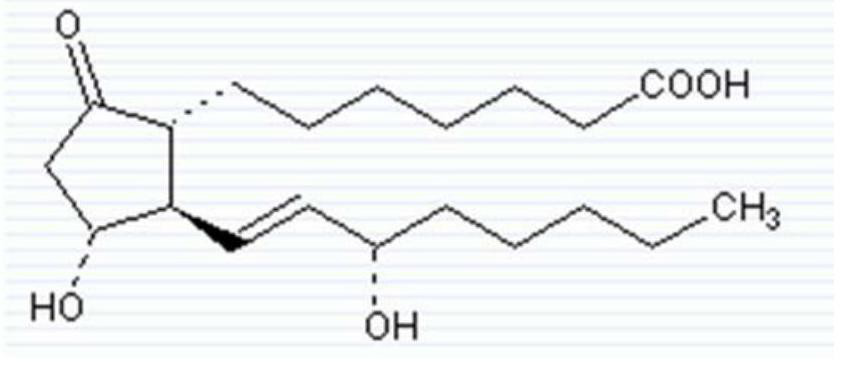
A kind of preparation method of alprostadil injection
A technology of alprostadil and injection, which is applied in the field of medicine, can solve the problems of reducing the stability of alprostadil emulsion, achieve the effects of stable encapsulation rate and drug content, high encapsulation rate, and little vascular stimulation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
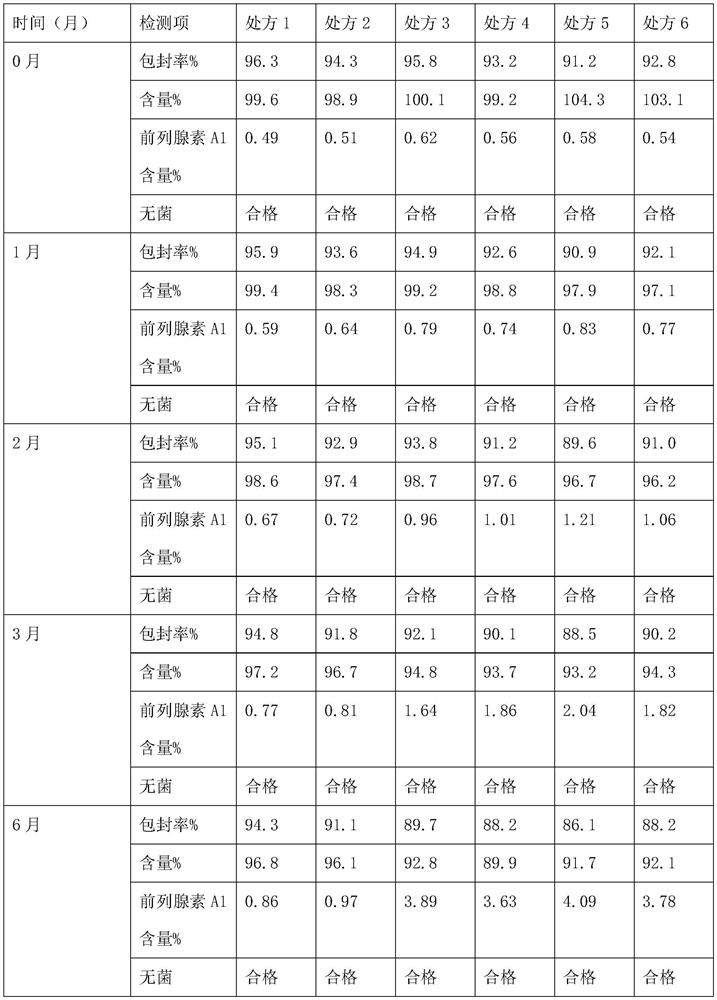
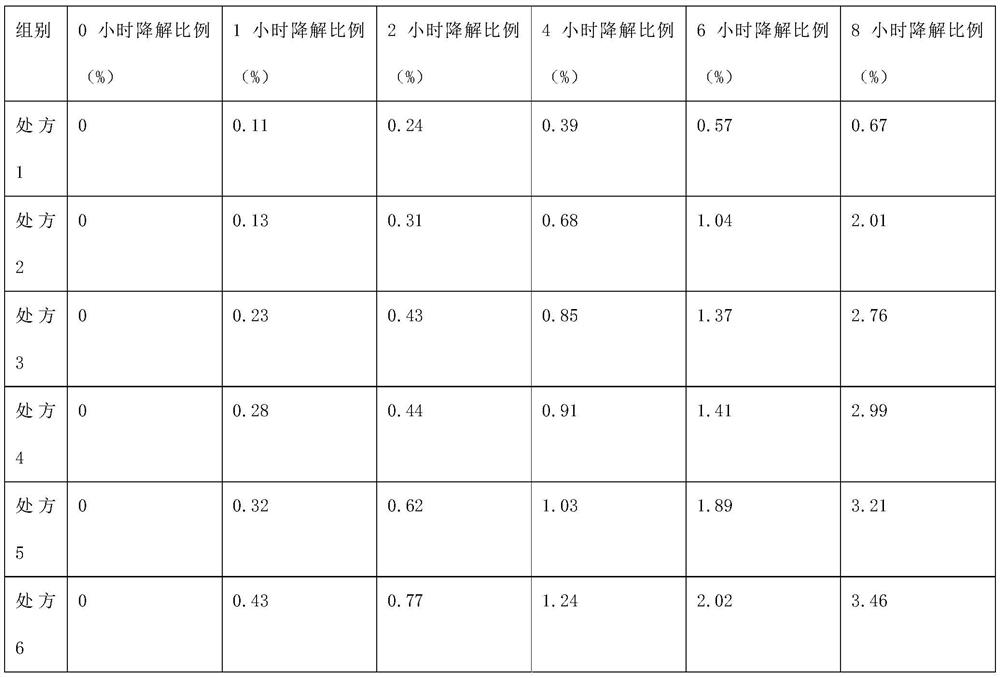
[0051] Embodiment 1: Emulsion injection prescription and preparation method thereof
[0052] Press the prescription of table 1, take each raw material by prescription respectively, (1) add 15mg phospholipid and polyethylene glycol 2000 after dissolving in the mixed solvent of ethanol and ether (volume ratio 1:1), add 5mg alprostadil and stir Dissolve, recover ethanol and ether under reduced pressure, and dry to obtain powder; (2) Mix soybean oil and medium-chain triglycerides and heat to 50°C, add 15g egg yolk lecithin and the powder prepared in step (1) in sequence, Under a high-shear dispersing emulsifier, high-speed shearing and dispersing for 15 minutes to prepare an oil phase; (3) adding glycerin to water for injection, and shearing and dispersing for 10 minutes under a high-shear dispersing and emulsifying machine to prepare an aqueous phase; (4) Under high-speed shear dispersion, slowly add the oil phase into the water phase, and then continue high-speed shear dispersio...
Embodiment 2
[0072] prescription:
[0073]
[0074] Preparation method: Weigh each raw material according to the prescription, (1) add 10 mg of egg yolk lecithin and 3 mg of polyethylene glycol 2000 into a mixed solvent of ethanol and ether (1:1 by volume) to dissolve, add 4 mg of alprostadil and stir Dissolve, recover ethanol and ether under reduced pressure, and dry to obtain powder; (2) Mix soybean oil and medium-chain triglycerides and heat to 45°C, add 10g of lecithin and the powder prepared in step (1) successively, Under the shear dispersing emulsifier, high-speed shearing and dispersing for 25 minutes to prepare an oil phase; (3) adding glycerin to water for injection, under a high-shear dispersing emulsifier, shearing and dispersing for 10 minutes to prepare an aqueous phase; ( 4) Under high-speed shear dispersion, slowly add the oil phase to the water phase, and then continue high-speed shear dispersion at 45°C for 20 minutes to prepare colostrum; (5) Add water for injection t...
Embodiment 3
[0076] prescription:
[0077]
[0078] Preparation method: Weigh each raw material according to the prescription, (1) add 12 mg egg yolk lecithin and 2 mg polyethylene glycol 2000 to ethanol for dissolution, add 4 mg alprostadil and stir to dissolve, recover ethanol under reduced pressure, and dry to obtain powder; (2) Mix soybean oil and medium-chain triglycerides and heat to 55°C, add 15g of egg yolk lecithin and the powder prepared in step (1) in sequence, and disperse under high-shear dispersing emulsifier for 25 minutes at high speed , prepared into an oil phase; (3) adding glycerin to water for injection, and shearing and dispersing for 10 minutes under a high-shear dispersing emulsifier to prepare an aqueous phase; (4) under high-speed shearing and dispersing, the oil phase was slowly Add it into the water phase, and then continue high-speed shear dispersion at 40°C for 20 minutes to prepare colostrum; (5) Add water for injection to the full amount of colostrum, adju...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com