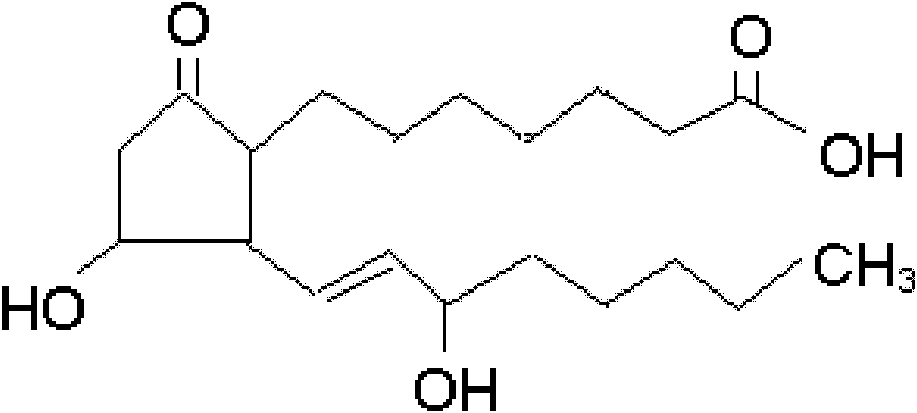
Alprostadil injection and preparation method thereof
The technology of dier injection and alprostadil is applied in the direction of medical formula, emulsion delivery, medical preparations containing active ingredients, etc., which can solve the inconvenience of transportation, production, storage and use, and the instability of alprostadil in water , the validity period is only 12 months, etc., to achieve good application prospects, enhance safety and stability, distinct creativity and practicability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Add 5mg alprostadil, 12g soybean lecithin (for injection) and 100g soybean oil into the container, and disperse with high shear for 15 minutes to dissolve to form an oil phase; get another container and add 850g water for injection, and 22g glycerin (for injection) ), 0.8g of poloxamer 188 was added and stirred at a low speed for 5 minutes to form a water phase, and the pH value of the water phase was adjusted to 5.5 with 25mM citric acid; After running, slowly add the oil phase, emulsify for 20 minutes to form colostrum, and set the volume to 1000ml. Put the colostrum into the feed port of the high-pressure micro-fluidic nano-disperser, and homogenize it for 3 times under the pressure of 18000-20000Psi. Filling, corking, capping. to a rotary water bath sterilizer, and sterilized at 126°C for 4 minutes (verified sterilization F 0 Value greater than 12, for the overkill method) to obtain.
Embodiment 2
[0059] Add 5mg alprostadil, 18g soybean lecithin (for injection) and 100g soybean oil into the container, and disperse with high shear for 15 minutes to dissolve to form an oil phase; get another container and add 850g water for injection, and 22g glycerin (for injection) ), 2g of Poloxamer 188 was added and stirred at low speed for 5 minutes to form a water phase, and the pH value of the water phase was adjusted to 5.5 with 25mM citric acid; Finally, slowly add the oil phase, emulsify for 20 minutes to form colostrum, and set the volume to 1000ml. Put the colostrum into the feed port of the high-pressure micro-jet nano-disperser, and homogenize it for 3 times under the pressure of 18000-20000Psi. Filling, corking, capping. to a rotary water bath sterilizer, and sterilized at 126°C for 4 minutes (verified sterilization F 0 Value greater than 12, for the overkill method) to obtain.
Embodiment 3
[0061] Add 10mg alprostadil, 18g soybean lecithin (for injection) and 100g soybean oil into the container, and disperse with high shear for 15 minutes to dissolve to form an oil phase; get another container and add 850g water for injection, and 22g glycerin (for injection) ), 2g of poloxamer 188 was added and stirred at a low speed for 5 minutes to form an aqueous phase, and the pH value of the aqueous phase was adjusted to be within 5.5 with 10mM citric acid; the aqueous phase was placed under the working head of a high-shear dispersing emulsifier, After running at high speed, slowly add the oil phase, emulsify for 20 minutes to form colostrum, and set the volume to 1000ml. Put the colostrum into the feed port of the high-pressure micro-fluidic nano-disperser, and homogenize it for 3 times under the pressure of 18000-20000Psi. Filling, corking, capping. to a rotary water bath sterilizer, and sterilized at 126°C for 4 minutes (verified sterilization F 0 Value greater than 12...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com