Method for controlling quality of alprostadil injection
A technology of alprostadil and prostaglandin, which is applied in the preparation of test samples, measuring devices, instruments, etc., can solve the problems of complex process, inaccurate analysis results, affecting analysis precision and accuracy, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0032] Preparation of internal standard solution Take about 25mg of β-naphthol, weigh it accurately, put it in a 50ml measuring bottle, add absolute ethanol to dissolve and dilute to the mark, measure 5ml, put it in a 50ml measuring bottle, add mobile phase to dilute to the mark, Shake well and serve.
[0033] The preparation of reference substance solution gets about 5 mg of alprostadil reference substance (in a phosphorus pentoxide decompression desiccator, dried at room temperature for 4 hours), accurately weighed, puts in a 50ml brown measuring bottle, adds dehydrated alcohol 10ml to dissolve, and Dilute to the mark, shake well, accurately measure 5ml to a 100ml measuring bottle, add mobile phase to dilute to the mark, shake well, accurately measure 1ml and 1ml of internal standard solution, put it in a 10ml measuring bottle, add mobile phase to dilute to the mark, Shake well as a reference solution.
[0034] Preparation of the test solution Precisely measure an appropria...
Embodiment 1
[0041] Prostaglandin A 1 Determination of
[0042] According to high performance liquid chromatography (Chinese Pharmacopoeia 2000 edition two appendix V D) determination.
[0043] 1. Chromatographic conditions
[0044] Instrument: Agilent 1100 HPLC
[0045] Chromatographic column: Kromasil C18, 4.6×250mm, 5μm
[0046] Mobile phase: 0.0067mol / L phosphate buffer: acetonitrile (74:26)
[0047] Flow rate: 1.4ml / min
[0048] Post-column reaction equipment: Waters 515 pump, Waters column thermostat
[0049] Post-column reaction solution: 1mol / L potassium hydroxide solution
[0050] Post-column reaction tube: polytetrafluoroethylene tube (φ0.5mm×10m)
[0051] Column temperature: 60°C Detection wavelength: 278nm Injection volume: 100μl
[0052] Flow rate: 0.3ml / min
[0053] 2. Reagent and test solution
[0054] Alprostadil reference substance (National Institute for the Control of Pharmaceutical and Biological Products)
[0055] Alprostadil raw material (raw material when ma...
Embodiment 2
[0102] Determination of PGE1 content
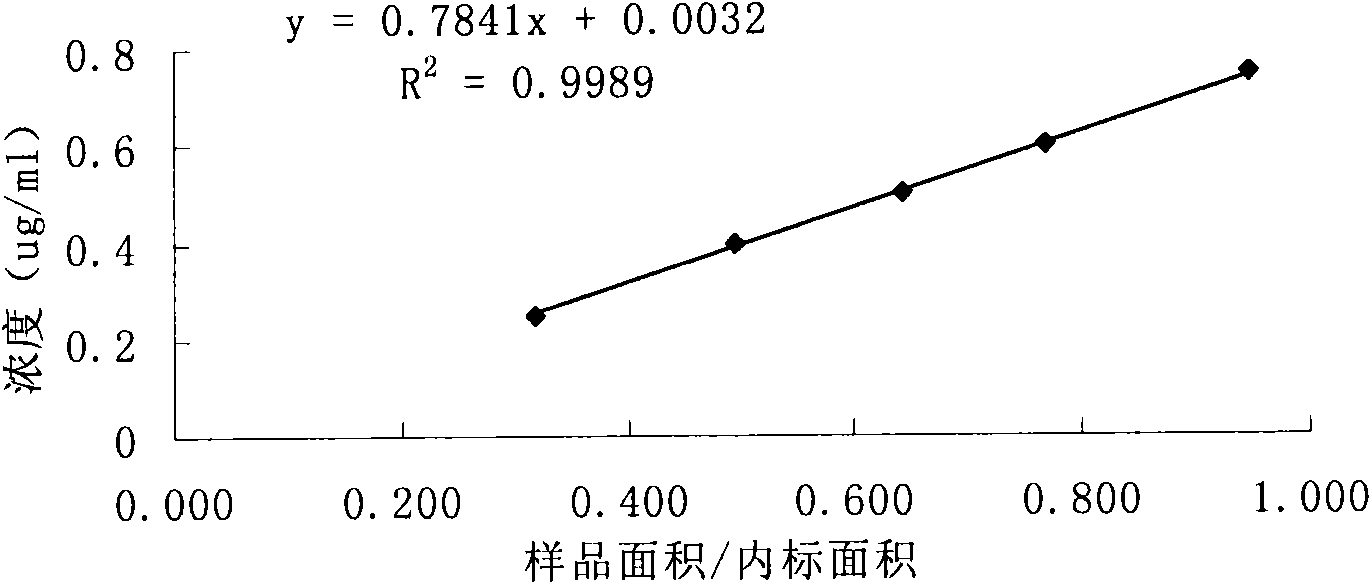
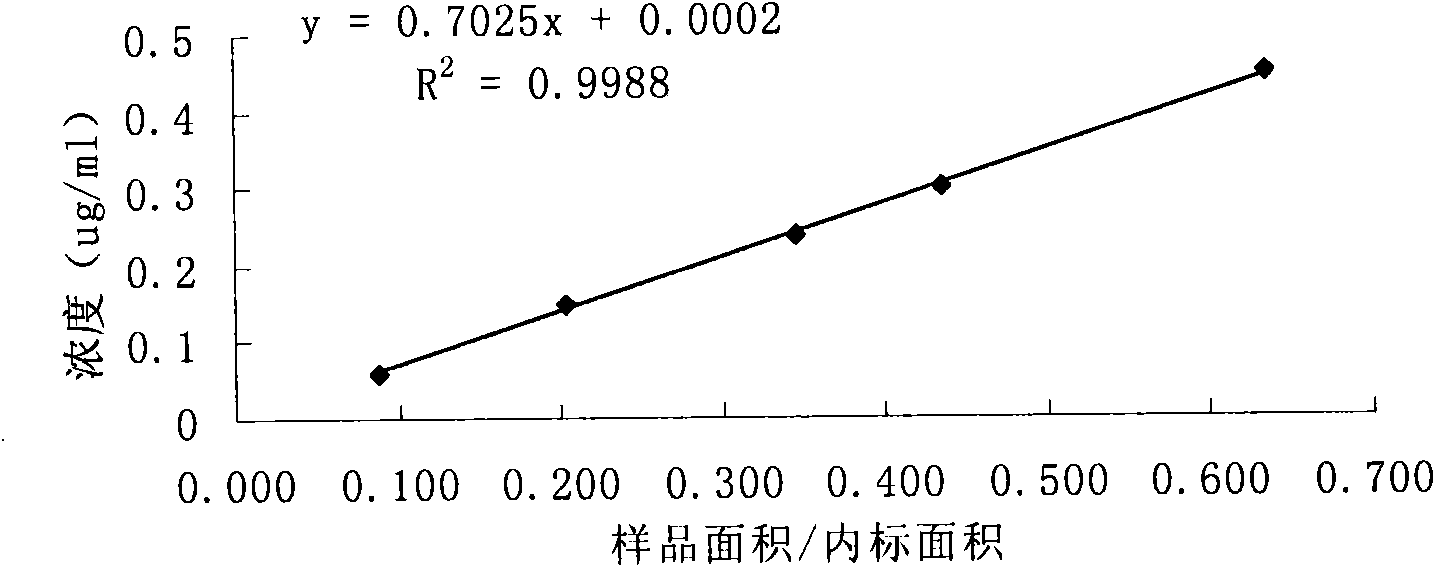
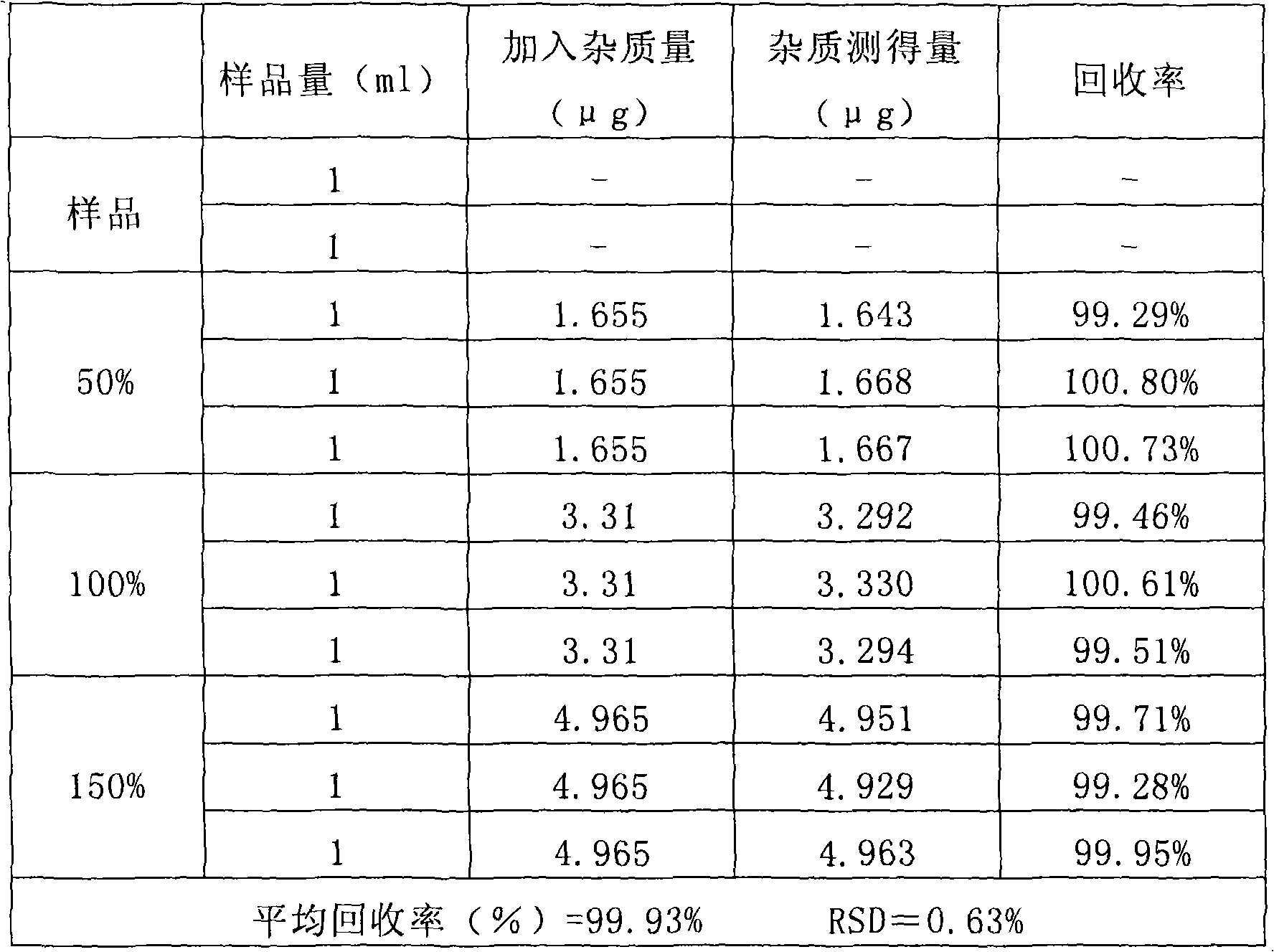
[0103] According to high performance liquid chromatography (Chinese Pharmacopoeia 2000 edition two appendix V D) determination. After system suitability test, precision test, linear regression test and recovery rate test, the results are satisfactory.
[0104] 1. Chromatographic conditions: the same as the detection method of prostaglandin A1 in Example 1
[0105] 2. System suitability test See the system suitability test under the item of prostaglandin A1 in Example 1.
[0106] 3. Precision test
[0107] Prepare according to the sample solution preparation method, repeat the measurement 6 times according to the content determination method, record the ratio of the main peak area to the internal standard peak area, and calculate the relative standard deviation. (See Table 4)
[0108] Table 4 precision test results
[0109]
[0110] Because the RSD<2.0%, the sample precision test results meet the requirements of the Pharmacopoeia m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com