Topical compositions for prostaglandin E1 delivery
A prostaglandin and composition technology, which is applied in the field of pharmaceutical composition for transdermal administration of prostaglandin drugs to patients, can solve problems such as instability, and achieve reduced skin irritation, high prostaglandin penetration and bioavailability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Example 1: Epidermal administration of prostaglandin E 1 Composition A
[0052] Composition A is prepared according to the following method, with 0.4 parts of prostaglandin E 1 (Alprostadil USP) was dissolved in 5 parts of ethanol to form part A, then 5 parts of dodecyl 2-(N,N-dimethylamino)propionate were mixed into the alcohol-prostaglandin E1 solution, followed by Parts of ethyl laurate.
[0053] Prepare Part B starting from a water / buffer solution at pH 5.5: by adding sufficient potassium phosphate monohydrate to pure water to obtain a 0.1M solution, with a strong base solution (1N sodium hydroxide) and a strong acid solution (1N phosphoric acid) Adjust the pH of the water / buffer solution to 5.5. The buffer solution comprises about 80 parts of the total composition.
[0054] Add 0.5 parts of ethyl laurate to the buffer solution, then disperse locust bean gum (powder) into the buffer solution, and homogenize with a homogenizer. Table 1 below lists the components....
Embodiment 2
[0060] Example 2: Epidermal administration of prostaglandin E 1 Composition B
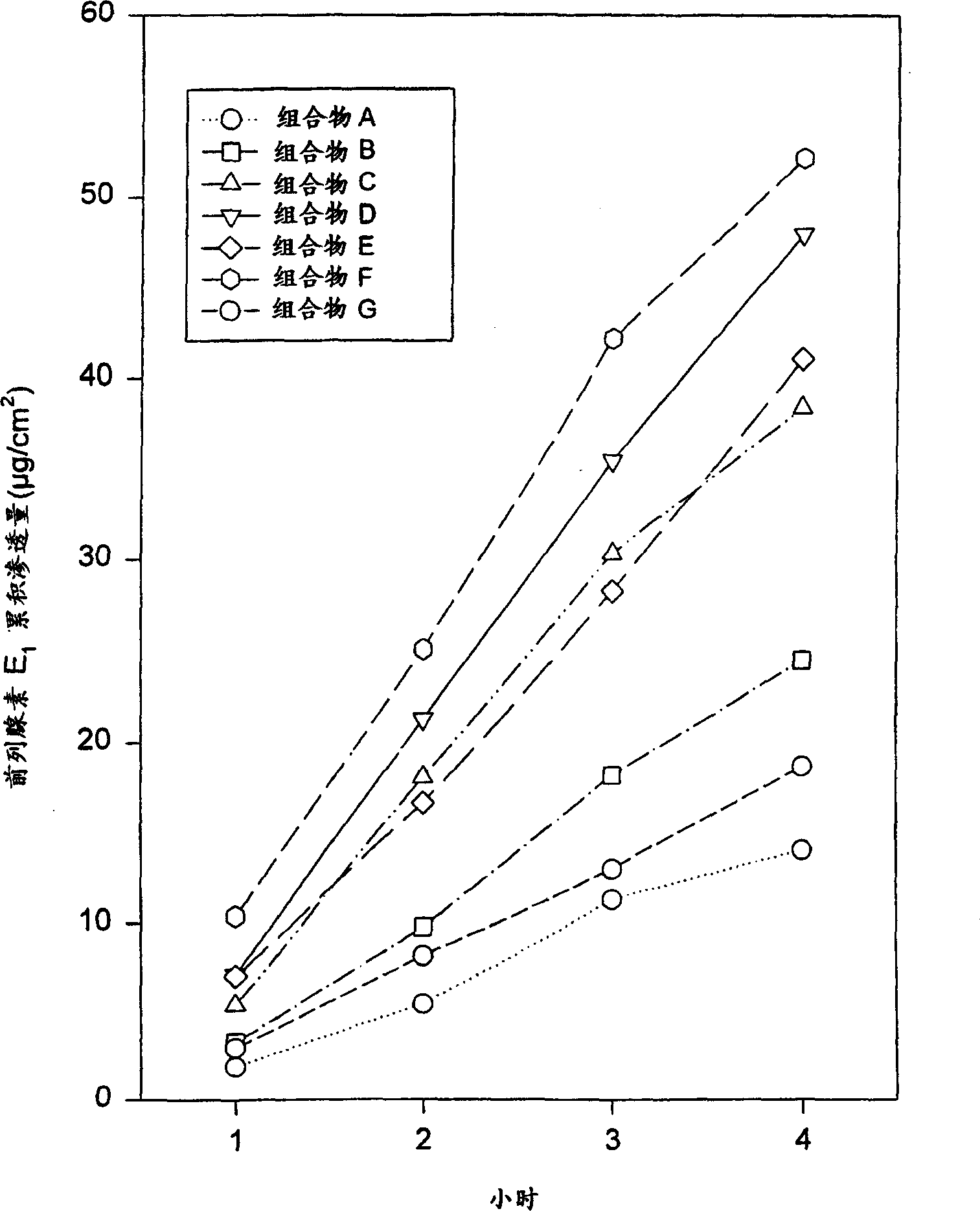
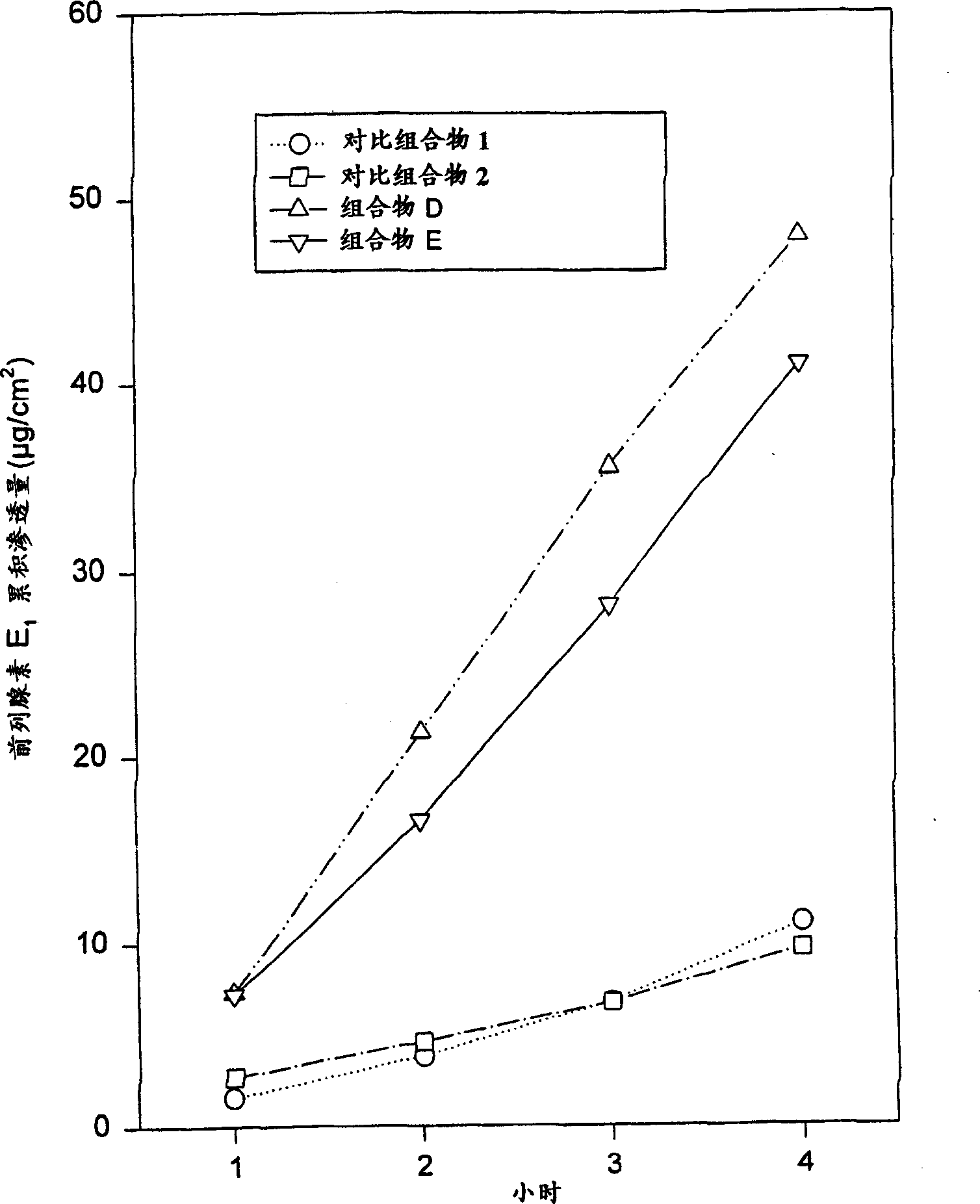
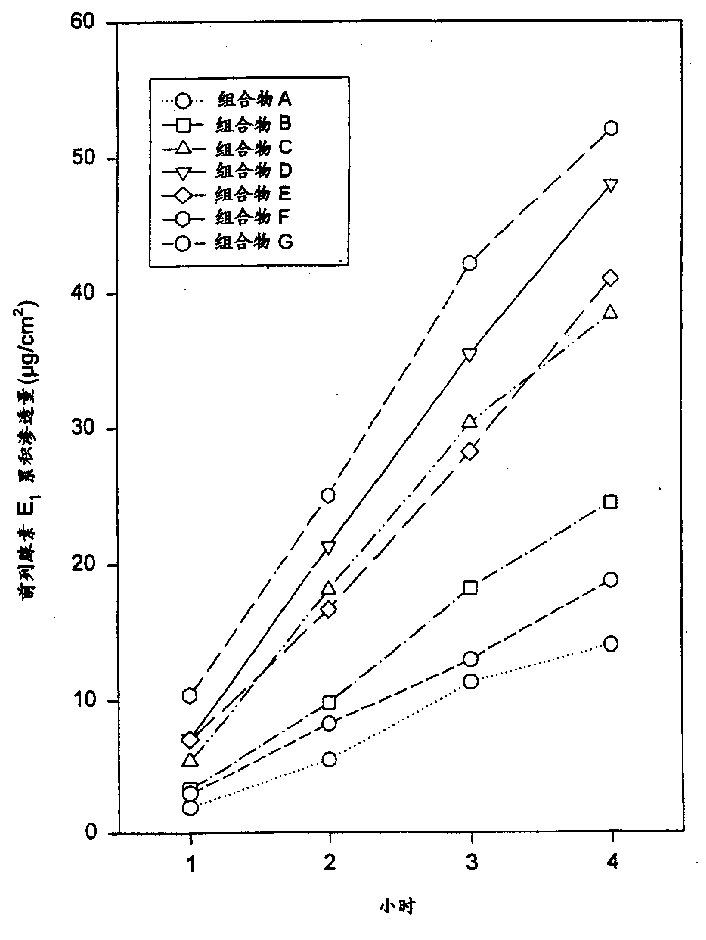
[0061] Composition B was prepared using the ingredients listed in Table 1. Composition B contains more prostaglandin E than composition A 1 , Composition B exhibited similar semi-solid homogeneity and homogeneous appearance despite increased drug dosage. Determination of prostaglandin E according to the method described in implementation 1. 1 permeability. Composition B provides prostaglandin E 1 The relatively rapid and constant delivery, the results are listed in Table 2 and figure 1 middle.
Embodiment 3
[0062] Example 3: Epidermal administration of prostaglandin E 1 Composition C
[0063] Composition C was prepared using the ingredients listed in Table 1 below. Composition C contains more prostaglandin E than compositions A or B 1 , increasing the amount of drug had little (or no) effect on uniformity and appearance, and was actually comparable to composition A or B. Still measure prostaglandin E according to the method described in embodiment 1 1 permeability. According to this test, composition C also provides prostaglandin E 1 The relatively rapid and constant delivery of , the results are listed in Table 2 below and figure 1 middle.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com