Prostaglandin compositions and method of treatment for male erectile dysfunction
An erectile dysfunction, prostaglandin technology, applied in drug combinations, sexual diseases, drug delivery, etc., can solve problems such as complication, unstable effort, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0091] Example 1: Topical Prostaglandin E 1 Composition A
[0092] Composition A was prepared as follows. by dissolving 0.4 parts prostaglandin E in 5 parts ethanol 1 (Alprostadil USP) Part A was prepared. In the next step, 5 parts of 2-(N,N-dimethylamino)-dodecyl propionate were mixed into the alcohol-prostaglandin E 1 solution, followed by the addition of 5 parts of ethyl laurate.
[0093] Part B was prepared starting with water / buffer at pH 5.5. Water / buffers were prepared by adding sufficient potassium phosphate monohydrate to purified water to produce a 0.1M solution. The water / buffer pH was adjusted to 5.5 with a strong base solution (1 N sodium hydroxide) and a strong acid (1 N phosphoric acid). Buffer represents 80 parts of the total composition.
[0094] Add 0.5 parts of ethyl laurate to the buffer. In the next step, locust bean gum (in powder form) was dispersed into the buffer and homogenized using a homogenizer. Table 1 below contains a list of the compone...
Embodiment 2
[0100] Example 2: Topical Prostaglandin E 1 Composition B
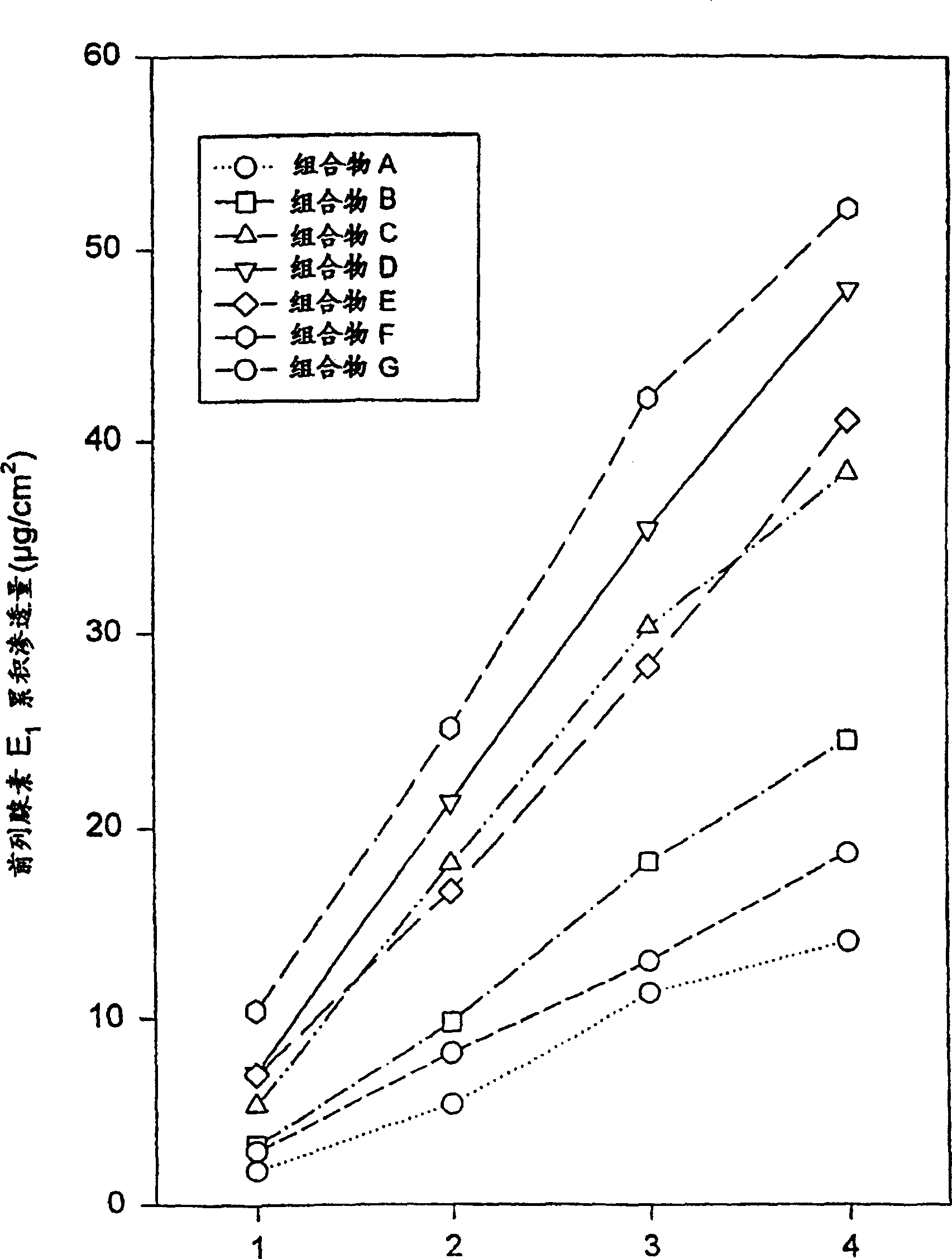
[0101] Composition B was prepared using the ingredients listed in Table 1. Composition B contains more prostaglandin E than composition A 1 . Composition B exhibited a similar semi-solid consistency and uniform appearance despite the increased drug dosage. Determination of prostaglandin E according to the method described in Example 1 1 permeability. Composition B provides prostaglandin E 1 The relatively rapid and constant delivery of , the results are listed in Table 2 below and image 3 middle.
Embodiment 3
[0102] Example 3: Topical Prostaglandin E 1 Composition C
[0103] Composition C was prepared using the ingredients listed in Table 1. Composition C contains more prostaglandin E than compositions A or B 1 . Increased drug levels had less (or no) effect on consistency and appearance and were actually comparable to compositions A or B. Still measure prostaglandin E according to the method described in embodiment 1 1 permeability. According to this test, composition C also provides prostaglandin E 1 The relatively rapid and constant delivery of , the results are listed in Table 2 below and image 3 middle.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com