Preparation method of alprostadil injection
A technology of dil injection and alprostadil, which is applied in the field of preparation of alprostadil injection, can solve the problem that sterilization filtration cannot be completed, refractory alprostadil injection sterilization filtration, and unfiltered emulsion cannot reach the surface of filter membrane and other problems, to achieve the effect of reducing the incidence of adverse reactions, reducing the content of degraded impurities and lysophospholipids, and shortening the time.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] For the convenience of examining the process, the present invention proposes a prescription as follows, but the process of the present invention is not limited to adapting to this specific prescription.
[0028] Alprostadil injection prescription
[0029] Alprostadil (PGE 1 ) 5mg
[0030] Refined soybean oil 100g
[0032] Oleic acid 1.8g
[0033] Glycerin 22.5g
[0034] Appropriate amount of sodium hydroxide
[0035] Add water for injection to 1000ml
[0036] Process: (1) Preparation of water phase: add glycerin to water to dissolve, heat to 55-70°C, and set aside;
[0037] (2) Preparation of the oil phase: heat the refined soybean oil to 55-70°C, add egg yolk lecithin, dissolve oleic acid, add alprostadil, stir to dissolve;
[0038] (3) Preparation of colostrum: Add the oil phase of step (2) into the water phase of step (1), at a temperature of 55-70° C., high-speed shear dispersion, with a shear rate of 8000 rpm, for 15 minutes, ...
Embodiment 2
[0043] Embodiment 2: the preparation temperature of colostrum
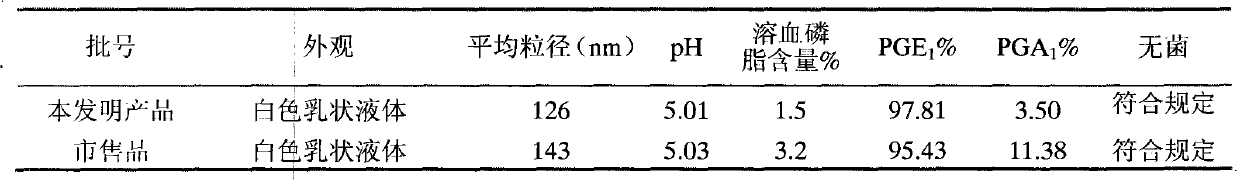
[0044] Set up three temperature inspection points respectively. Under the same conditions of other processes, emulsification effect, prostaglandin E 1 (PGE 1 ) and the degradation product prostaglandin A 1 (PGA 1 ) content as an index, and the investigation was carried out at 50, 55, 70, and 90°C respectively, and the results are shown in Table 1.
[0045] Table 1 Investigation of emulsifier dispersion temperature (made into 100mL)
[0046]
[0047] The test results show that the emulsification effect at 50°C is poor, and there are obvious oil droplets, which is not suitable for the next step of high-pressure homogenization; the content measurement results show that too high dispersion temperature is not conducive to the stability of the drug. Comprehensive consideration, choose the initial The emulsification temperature of 55-70°C can meet the quality requirements of the product.
Embodiment 3
[0048] Embodiment 3: Selection of high-pressure milk homogenization temperature
[0049] Get the colostrum prepared under the condition of 55 ℃ among the embodiment 2, have investigated the finished product after high-pressure homogenization in two kinds of temperature ranges, with average particle diameter, prostaglandin E 1 and prostaglandin A 1 content as an indicator. The results are shown in Table 2 below.
[0050] Particle size distribution determination method: take a sample, dilute it to 5000 times with purified water (filtered through a 0.22 μm microporous membrane), mix well, and use a dynamic laser scattering particle size analyzer as the test solution.
[0051] Table 2 Investigation of high-pressure milk uniform dispersion temperature (made into 100mL)
[0052]
[0053] The results showed that, compared with 25°C, the high-pressure homogenization temperature at 65°C could obtain a fat emulsion with smaller particle size and uniform distribution, but at the sa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com