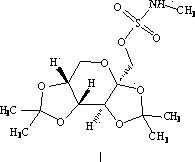
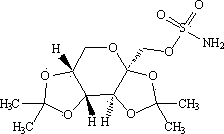
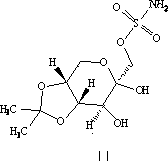
Topiramate granule and preparation method thereof
A technology of topiramate and granules, applied in the field of topiramate granules and their preparation, to achieve the effects of increasing compliance, improving bioavailability and improving water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] The preparation of embodiment 1 topiramate granules
[0039] prescription:
[0040] composition 100mg / bag (1000 bags) Proportion Topiramate 100g 10% β-cyclodextrin 370g 37% microcrystalline cellulose 330g 33% Croscarmellose 70g 7% Hypromellose 50g 5% Neohesperidin Dihydrochalcone 80g 8% gross weight 1000g 100%
[0041] Preparation Process:
[0042] ①. Dry topiramate, cyclodextrin and other excipients at 50-60°C for 5 hours, pass through a 100-mesh sieve for later use;
[0043] ②. Weigh the cyclodextrin according to the prescription amount to make a saturated aqueous solution at 50-60°C, then place it in a high-speed mixer, start stirring, and the rotation speed is 750r / min-2000r / min;
[0044]③. Dissolve the prescribed amount of topiramate in a small amount of 50% ethanol aqueous solution, and then slowly add it dropwise into the above-mentioned saturated cyclodextrin aqueous solution. After the addition...
Embodiment 2
[0047] The preparation of embodiment 2 topiramate granules
[0048] prescription:
[0049] composition 100mg / bag (1000 bags) Proportion Topiramate 100g 10% β-cyclodextrin 370g 37% lactose monohydrate 280g 28% Low-substituted hydroxypropyl cellulose 100g 10% Sodium carboxymethyl cellulose 90g 9% Neohesperidin Dihydrochalcone 60g 6% gross weight 1000g 100%
[0050] Preparation Process:
[0051] ①. Dry topiramate, cyclodextrin and other excipients at 50-60°C for 5 hours, pass through a 100-mesh sieve for later use;
[0052] ②. Weigh the cyclodextrin according to the prescription amount to make a saturated aqueous solution at 50-60°C, then place it in a high-speed mixer, start stirring, and the rotation speed is 750r / min-2000r / min;
[0053] ③. Dissolve the prescribed amount of topiramate in a small amount of 50% ethanol aqueous solution, and then slowly add it dropwise into the above-mentioned saturated cyclodextr...
Embodiment 3
[0056] The preparation of embodiment 3 topiramate granules
[0057] prescription:
[0058] composition 100mg / bag (1000 bags) Proportion Topiramate 100g 10% β-cyclodextrin 380g 38% pregelatinized starch 260g 26% Low-substituted hydroxypropyl cellulose 90g 9% Sodium carboxymethyl cellulose 90g 9% Neohesperidin Dihydrochalcone 80g 8% gross weight 1000g 100%
[0059] Preparation Process:
[0060] ①. Dry topiramate, cyclodextrin and other excipients at 50-60°C for 5 hours, pass through a 100-mesh sieve for later use;
[0061] ②. Weigh the cyclodextrin according to the prescription amount to make a saturated aqueous solution at 50-60°C, then place it in a high-speed mixer, start stirring, and the rotation speed is 750r / min-2000r / min;
[0062] ③. Dissolve the prescribed amount of topiramate in a small amount of 50% ethanol aqueous solution, and then slowly add it dropwise into the above-mentioned saturated cyclodextr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com