Preparation method of potassium magnesium aspartate freeze dried powder injection
A technology of potassium magnesium aspartate freeze-dried powder and aspartic acid, applied in the field of freeze-dried powder injection, can solve the problems of unqualified rate of clarity, incomplete removal of impurities, influence on clinical application, etc., and achieve improved clarity , good solubility, reduced irritation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
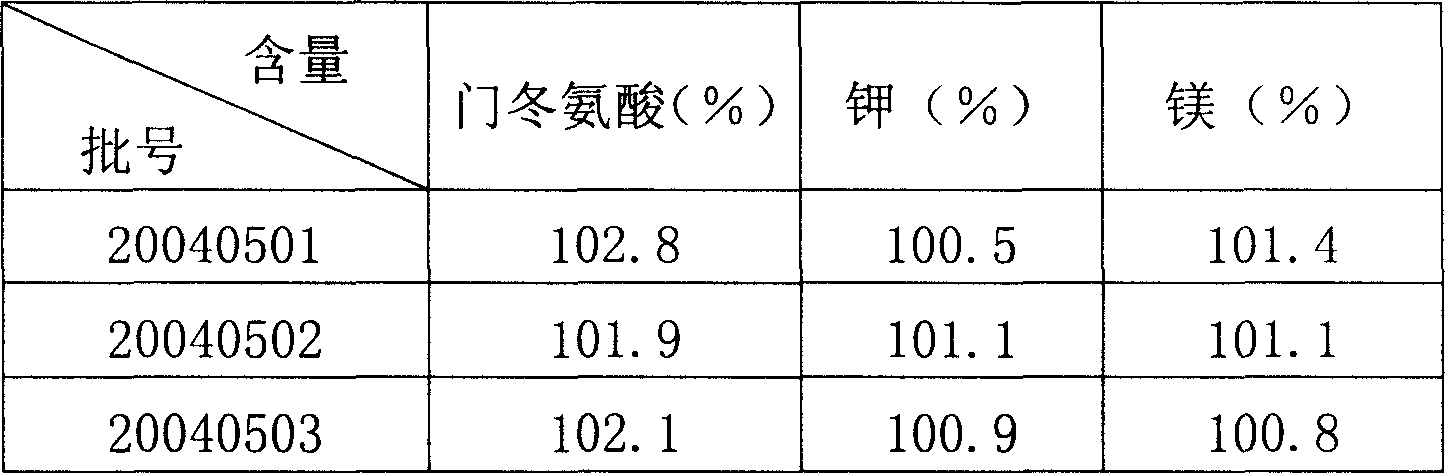
[0025] 1) Take 75% fresh water for injection at 85°C of the prepared amount, add aspartic acid in the prescription and stir to dissolve, then add magnesium oxide in the prescription, ultrasonicate for 60 minutes, refrigerate at 4°C for 12 hours, use Ultrafiltration membrane ultrafiltration to obtain filtrate I.
[0026] 2) Take 30% fresh water for injection at 90°C of the prepared amount, add potassium hydroxide and mannitol in the prescription to dissolve, add 0.1% activated carbon, heat and boil for 15 minutes, cool down to 40°C in circulation, filter with 0.45 μm microporous Membrane decarbonization and filtration to obtain filtrate II.
[0027] 3) Add the above medicinal solution II to medicinal solution I, mix evenly, constant volume, adjust pH to 5.8, after passing the inspection of the intermediate product, filter the medicinal solution through a terminal 0.22 μm filter membrane to sterilize and filter, and then fill it in penicillin in dark place bottle, refrigerated ...
Embodiment 2
[0029] 1) Take 80% fresh water for injection at 90°C of the prepared amount, add aspartic acid in the prescription and stir to dissolve, then add magnesium oxide in the prescription, treat with ultrasound for 30 minutes, refrigerate at 6°C for 24 hours, and use water with a molecular weight cut-off of 10,000 Ultrafiltration membrane ultrafiltration to obtain filtrate I.
[0030] 2) Take 25% fresh water for injection at 85°C of the prepared amount, add potassium hydroxide and mannitol in the prescription to dissolve, add 0.05% activated carbon, heat and boil for 30 minutes, cool down to 30°C in circulation, filter through 0.45μm microporous Membrane decarbonization and filtration to obtain filtrate II.
[0031] 3) Add the above medicinal solution II to medicinal solution I, mix evenly, constant volume, and adjust pH to 6.3. After passing the inspection of the intermediate product, the medicinal solution is sterilized and filtered through a terminal 0.22 μm filter membrane, and ...
Embodiment 3
[0033] 1) Take 70% fresh water for injection at 80°C of the prepared amount, add aspartic acid in the prescription and stir to dissolve, then add magnesium oxide in the prescription, ultrasonicate for 40 minutes, refrigerate at 0°C for 24 hours, and use water with a molecular weight cut-off of 8000 Ultrafiltration membrane ultrafiltration to obtain filtrate I.
[0034] 2) Take 10% fresh water for injection at 80°C of the prepared amount, add potassium hydroxide and mannitol in the prescription to dissolve, add 0.3% activated carbon, heat and boil for 20 minutes, cool down to 35°C in circulation, filter with 0.45 μm microporous Membrane decarbonization and filtration to obtain filtrate II.
[0035] 3) Add the above medicinal solution II to medicinal solution I, mix evenly, constant volume, and adjust pH to 6.0. After passing the inspection of the intermediate product, filter the medicinal solution through a terminal 0.22 μm filter membrane to sterilize and filter it, and then f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com