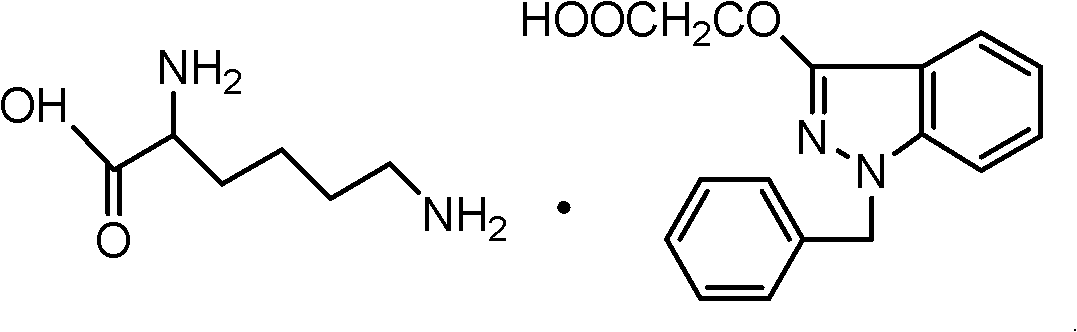
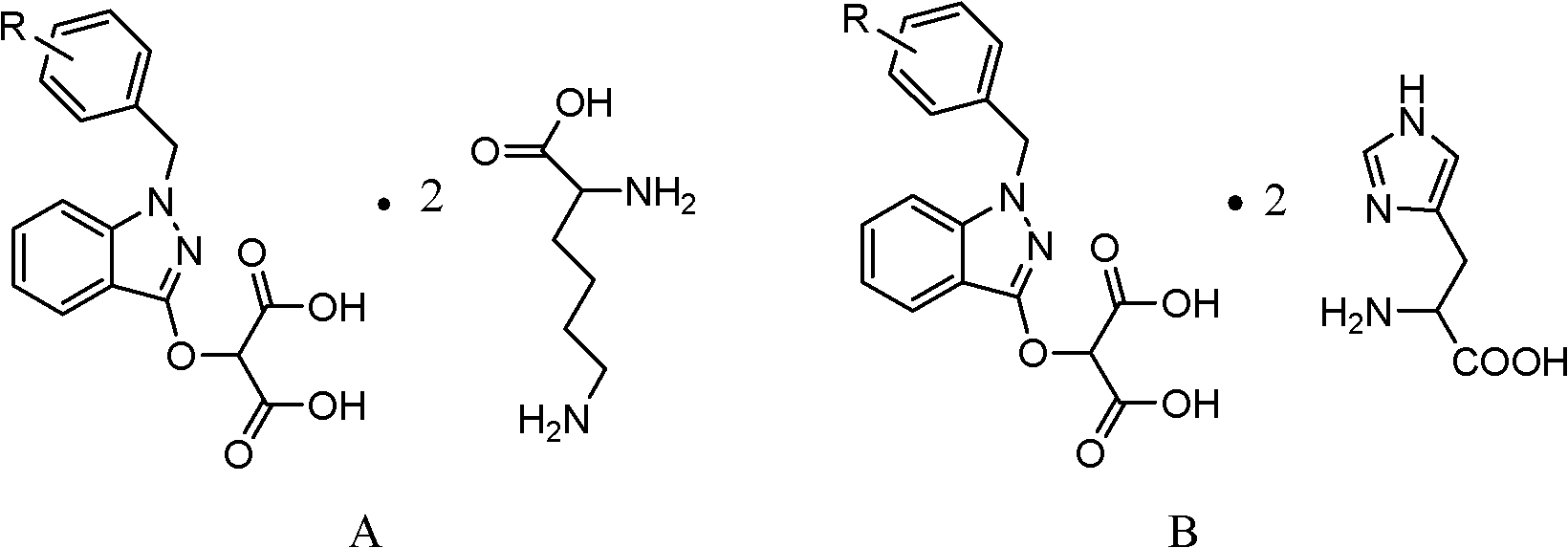
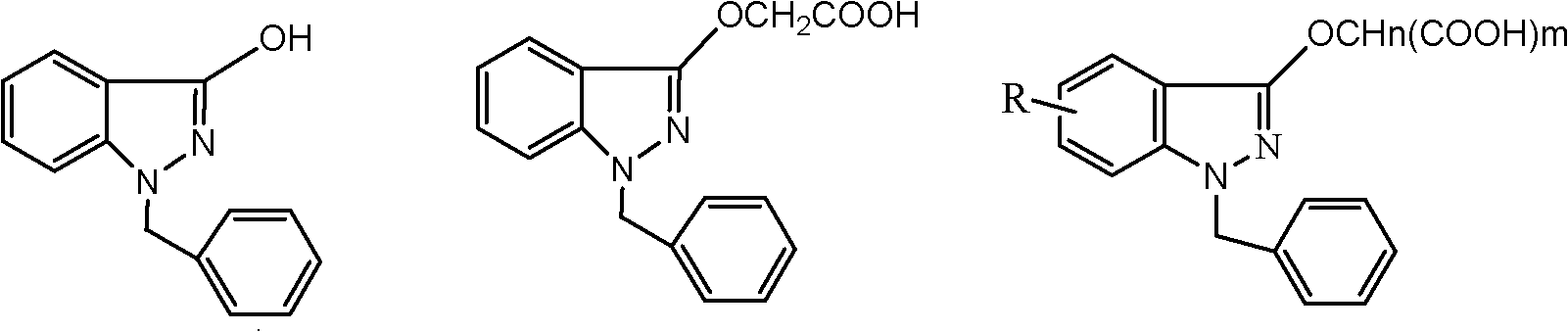
Process for refining bendazac lysine and analogs thereof
A technology of analogues, bentaxic acid, which is applied in the field of chemical drug preparation, can solve problems such as poor removal of impurities, impact on the quality of bentaxaric acid, and products that do not meet quality requirements, achieving short reaction time and low impurity Less, good crystal form effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment 1: the comparison of the implementation effect of salt-forming solvent and recrystallization solvent
[0041] 1. Comparison of salt-forming solvents:
[0042] 141g (0.5mol) of bentacic acid (0.5mol) and 73g (0.5mol) of L-lysine were weighed in 5 parts respectively, added in the reactor successively, and then respectively measured 90% (v / v) methanol, ethanol, acetone, Aqueous solutions of tetrahydrofuran and acetonitrile were sequentially added to the reactor, stirred, heated to reflux for 10 minutes, filtered hot, the filtrate was cooled to room temperature, filtered, and the filter cake was dried and weighed to obtain a crude product. The relevant experimental data are shown in Table I.
[0043] Table I
[0044]
[0045] Data in table 1 shows: compare two kinds of polar protic solvents of methanol and ethanol, use acetone, THF, acetonitrile three kinds of polar aprotic solvents in the salt-forming step, the related substance of gained product is low, an...
Embodiment 2
[0054] Embodiment 2: the comparison of salt-forming solvent concentration
[0055] 282 grams (1mol) of benzylic acid, 146 grams (1mol) of L-lysine, and the aqueous solution of acetonitrile were placed in the reactor together, stirred, heated to reflux for 10 minutes, heated and filtered, the filtrate was cooled to room temperature, filtered, and The filter cake was dried to obtain the crude product, which was weighed. The relevant experimental data of different aqueous solutions of acetonitrile (v / v) are shown in Table IV.
[0056] Table IV
[0057]
[0058] Data in Table IV shows that when the acetonitrile concentration is 90-95% (v / v), the volume of the reaction solution is moderate, and the related substances of the resulting product are low, the content is high, and the productive rate is high.
Embodiment 3
[0059] Embodiment 3. Comparison of recrystallization solvent concentration
[0060] Weigh the crude product obtained in Experiment 6-3 in the previous step, weigh 4 parts, each 100g, add to the reactor respectively, add aqueous solutions of ethanol with different concentrations (v / v), stir, heat and reflux for 10 minutes, and heat filter , the filtrate was naturally cooled to room temperature, placed for 12 hours, filtered, washed with absolute ethanol, dried, collected, and weighed. The experimental data obtained are shown in Table V.
[0061] Table V
[0062]
[0063] The data in Table V shows that: when the ethanol concentration is 91-94% (v / v), the volume of the reaction solution is moderate, the related substances of the obtained product are low, the content is high, and the yield is high.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com