Synthesis method of (S) (-)-amisulprideD-(-)-tartrate
The technology of a kind of amisulpride and synthetic method is applied in the field of synthesis of -amisulpride D--tartrate, which can solve the problems of large amount of waste liquid, large amount of synthetic solvent, high toxicity, etc. The effect of less dosage and high total yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
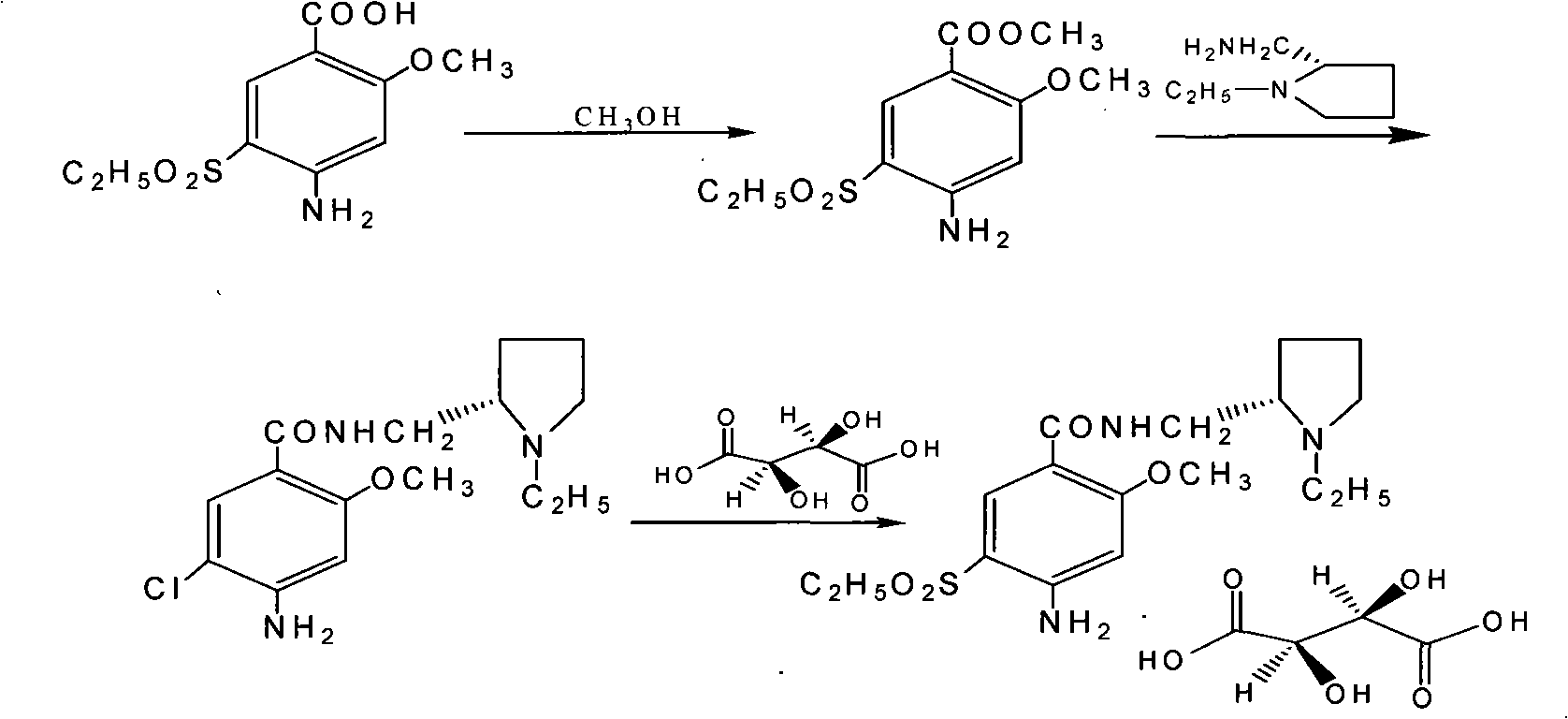
[0035] In the three-necked flask, 300 g of methanol, 10 g of concentrated sulfuric acid, and 100 g of 2-methoxy-4-amino-5-ethanesulfonylbenzoic acid were sequentially added, and the temperature was raised to reflux for 6 hours. Under ice water cooling, add 10% sodium carbonate solution dropwise to adjust the pH. When the pH is 8, cool and stir with ice water for 1 hour, filter the filter cake and wash it with tap water, and dry the filter cake at 60°C to obtain an off-white solid, which is the ester. compounds. Yield: 93%, melting point: 155-165°C;
[0036] In the three-necked flask, add 60g of glycerol, 54g of (S)(-)-1-ethyl-2-aminomethylpyrrole, and 90g of esterified product in sequence; after adding, stir evenly, and heat up to 80-90°C for 12 hours , the reaction was completed, lowered to 0-5 ° C and stirred for 3 hours, suction filtered (the mother liquor was collected separately), the filter cake was washed with a small amount of cold ethanol, and drained, the filter cak...
Embodiment 2
[0040] In the three-necked flask, 400 g of methanol, 15 g of concentrated sulfuric acid, and 100 g of 2-methoxy-4-amino-5-ethanesulfonylbenzoic acid were sequentially added, and the temperature was raised to reflux for 8 hours. Under ice water cooling, add 10% sodium carbonate solution dropwise to adjust the pH. When the pH is 7.5, cool and stir with ice water for 1.5 hours, filter the filter cake and wash it with tap water. Dry the filter cake at 60°C to obtain an off-white solid, namely For esterification. Yield: 94.5%, melting point: 155-165°C;
[0041] In the there-necked flask, sequentially add 60g of glycerol, 54g of (S)(-)-1-ethyl-2-aminomethylpyrrole, and 90g of esterified product; stir evenly after adding, heat up to 90-100°C and react for 9 hours , the reaction was completed, lowered to 0-5 ° C and stirred for 3 hours, suction filtered (the mother liquor was collected separately), the filter cake was washed with a small amount of cold ethanol, and drained, the filte...
Embodiment 3
[0045]In the three-neck flask, add 350 g of ethanol, 12 g of concentrated sulfuric acid, and 100 g of 2-methoxy-4-amino-5-ethanesulfonylbenzoic acid in sequence, heat up to reflux for 6 hours, and cool down to room temperature, and a large amount of solids precipitate out. Under ice-water cooling, add 10% sodium carbonate solution dropwise to adjust the pH. When the pH is 8.5, cool and stir with ice water for 1 hour, filter the filter cake and wash it with tap water. Dry the filter cake at 60°C to obtain an off-white solid, namely For esterification. Yield 91%;
[0046] In the three-necked flask, add ethylene glycol 60g, (S)(-)-1-ethyl-2-aminomethylpyrrole 54g, and esterified product 90g; after adding, stir evenly, heat up to 70-80°C for 16 hours , the reaction was completed, lowered to 0-5 ° C and stirred for 3 hours, suction filtered (the mother liquor was collected separately), the filter cake was washed with a small amount of cold ethanol, and drained, the filter cake was...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com