Synthesis method for amisulpride
A synthetic method, the technology of amisulpride, applied in the field of synthesis of pharmaceutical intermediates, can solve the problems of expensive and toxic reagents, numerous steps, and low yield, and achieve the effects of high atom utilization, simple equipment, and short routes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
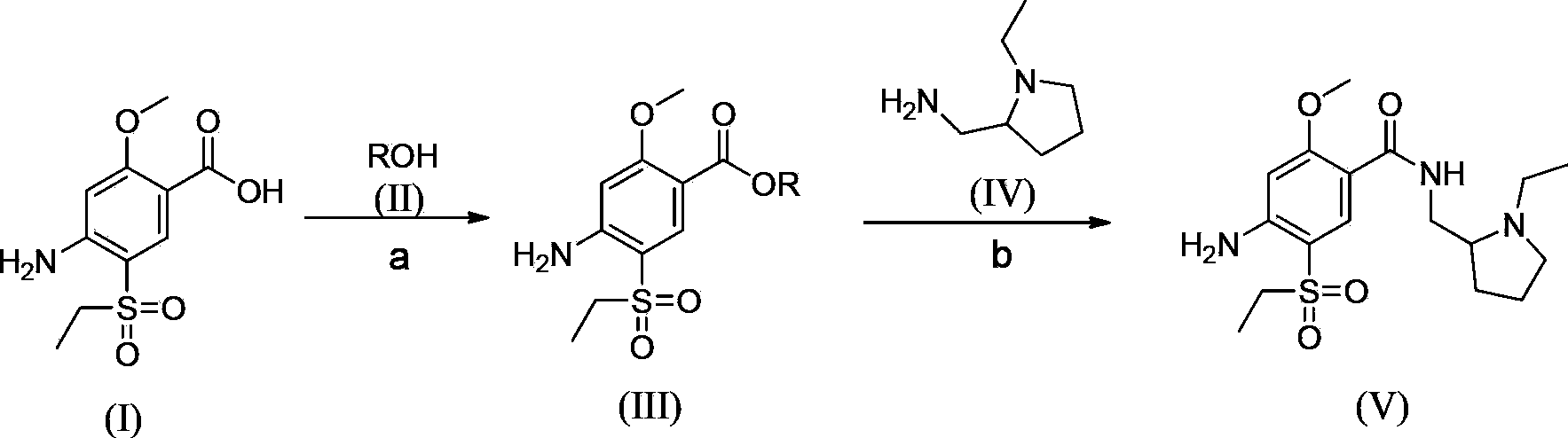
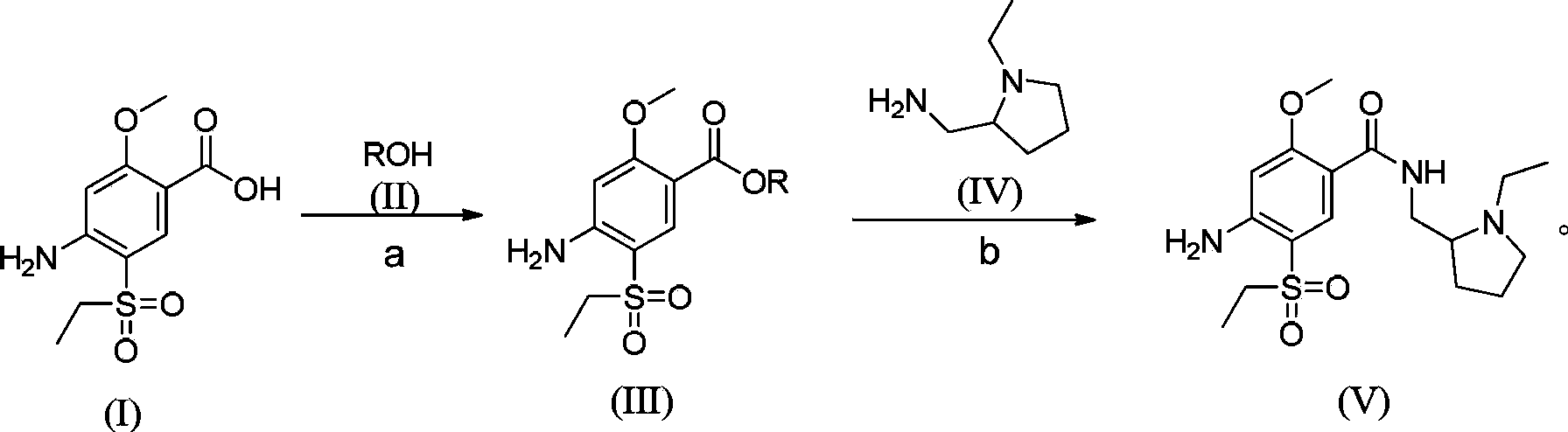
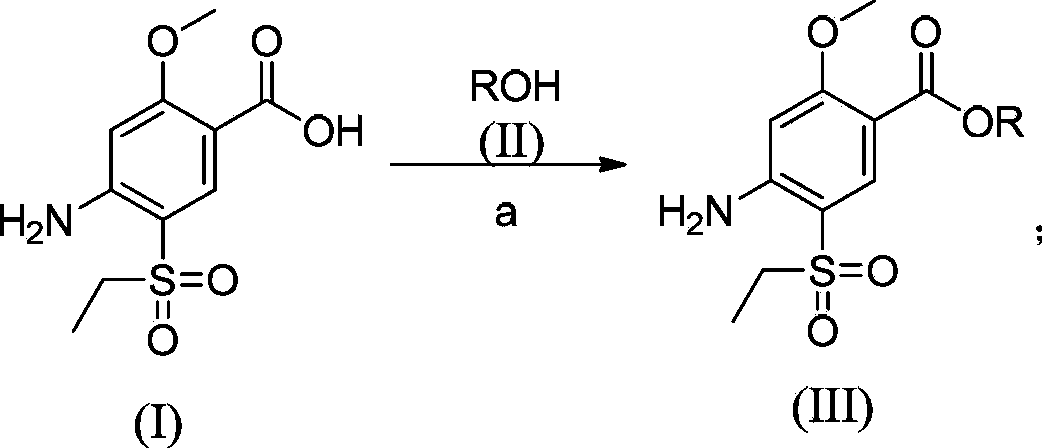
[0047]In a 10L four-neck flask, add 1kg of 2-methoxy-4-amino-5-ethanesulfonylbenzoic acid and 3kg of methanol in sequence, stir mechanically (100 rpm, internal temperature 30°C), and slowly Slowly add 300g of thionyl chloride, raise the temperature to reflux, reflux for 5 hours, cool the reaction system to 0°C, adjust the pH value to 9 with saturated sodium carbonate aqueous solution, add water to dilute to 4L (containing the volume of sodium carbonate aqueous solution), and filter with suction. The filter cake was dried at 50°C to obtain a light yellow solid, yield: 97.5%, purity: 97.7%; in a three-necked flask, add 1.2kg of esterified product, 675g of N-ethyl-2-aminopyrrole, and 1.2kg of isopropanol in sequence Stir mechanically, heat to an internal temperature of 80-85°C, react for 28 hours, when the temperature drops to 40-50°C, add 3L of water, stir at room temperature for 10 hours, a white solid precipitates, then stir at 0-5°C for 3 hours, filter, After washing with wat...
Embodiment 2
[0049] In a 10L four-necked flask, add 1kg of 2-methoxy-4-amino-5-ethanesulfonylbenzoic acid and 2.5kg of methanol in sequence, stir mechanically (100 rpm, internal temperature 30°C), and Slowly add 250g of thionyl chloride, raise the temperature to reflux, the internal temperature is 68°C, reflux for 7.5 hours, cool the reaction system to 0°C, adjust the pH value to 8 with saturated aqueous sodium carbonate solution, dilute to 4L with water (containing aqueous sodium carbonate volume), suction filtration, and the filter cake was dried at 50°C to obtain a light yellow solid, yield: 98%, purity: 95.2%;,
[0050] In the three-necked flask, add 1.2kg of esterified product, 675g of N-ethyl-2-aminopyrrole, and 1.2kg of isopropanol in sequence, stir mechanically, heat to an internal temperature of 95-100°C, react for 20 hours, and cool down to 40-50°C After a while, add 3L of water, stir at room temperature for 12 hours, a white solid precipitates, then stir at 0-5°C for 3 hours, fi...
Embodiment 3
[0052] In a 10L four-neck flask, add 1kg of 2-methoxy-4-amino-5-ethanesulfonylbenzoic acid and 3.5kg of ethanol in sequence, stir mechanically (rotating speed: 100 rpm, internal temperature: 30°C), at room temperature Slowly add 100g of thionyl chloride, raise the temperature to reflux, the internal temperature is 68°C, reflux for 5 hours, cool the reaction system to 0°C, adjust the pH value to 10 with saturated sodium carbonate aqueous solution, add water to 4L (containing sodium carbonate aqueous solution volume), suction filtration, the filter cake was dried at 50°C to obtain a light yellow solid, yield: 89.3%, purity: 96.1%;
[0053] In the three-necked flask, sequentially add 1.2kg of esterified product, 675g of N-ethyl-2-aminopyrrole, and 1.2kg of isopropanol with mechanical stirring, heat to an internal temperature of 100-105°C, react for 40 hours, and cool down to 40-50°C After a while, add 3L of water, stir at room temperature for 10 hours, a white solid precipitates,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com