Preparation method of amisulpride
An amisulpride and amino technology, applied in the field of drug synthesis, can solve the problems of affecting the quality of amisulpride finished products, high viscosity, inconvenient use and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
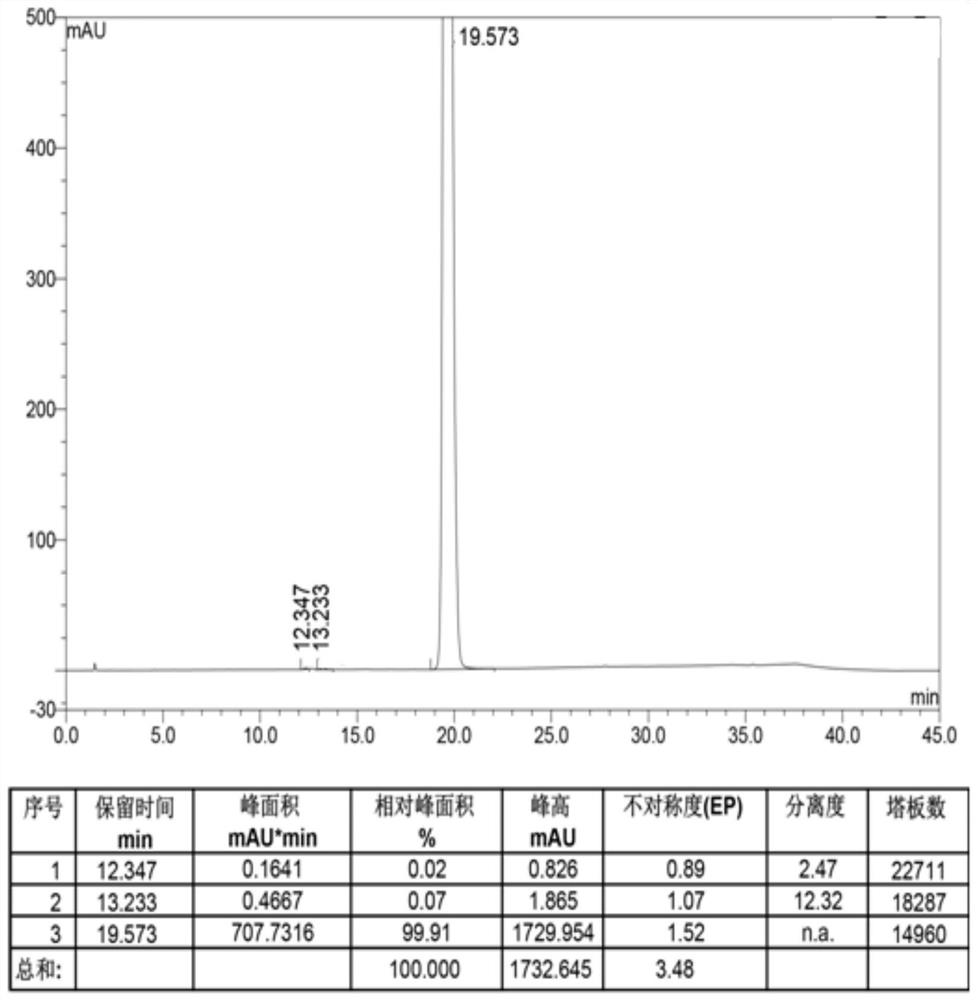
[0035]With 106.5g 4-amino-2-methoxy-5-ethylsulfonylbenzoic acid methyl ester (methyl amiate), 99.8g N-ethyl-2-aminomethylpyrrolidine, 156.0g concentration is Add 30%wt sodium methoxide methanol solution into 1065ml of methanol, after stirring evenly, raise the temperature to 65°C and keep it warm for 20h. After the reaction is completed, concentrate the reaction solution until the solvent no longer drips out, and then add 530ml of water, stirred at 10-20°C for 4h, precipitated a white solid, filtered, added 530ml of water to the obtained white solid for beating for 1h, filtered, washed, and air-dried at 50°C for 15h to obtain White solid amisulpride 136.1g, HPLC purity 99.91%, yield 94.5%, 4-amino-2-methoxy-5-ethylsulfonylbenzoic acid methyl ester (methyl amiate) residue 0.07%, The total amount of other impurities is 0.02%.
Embodiment 2
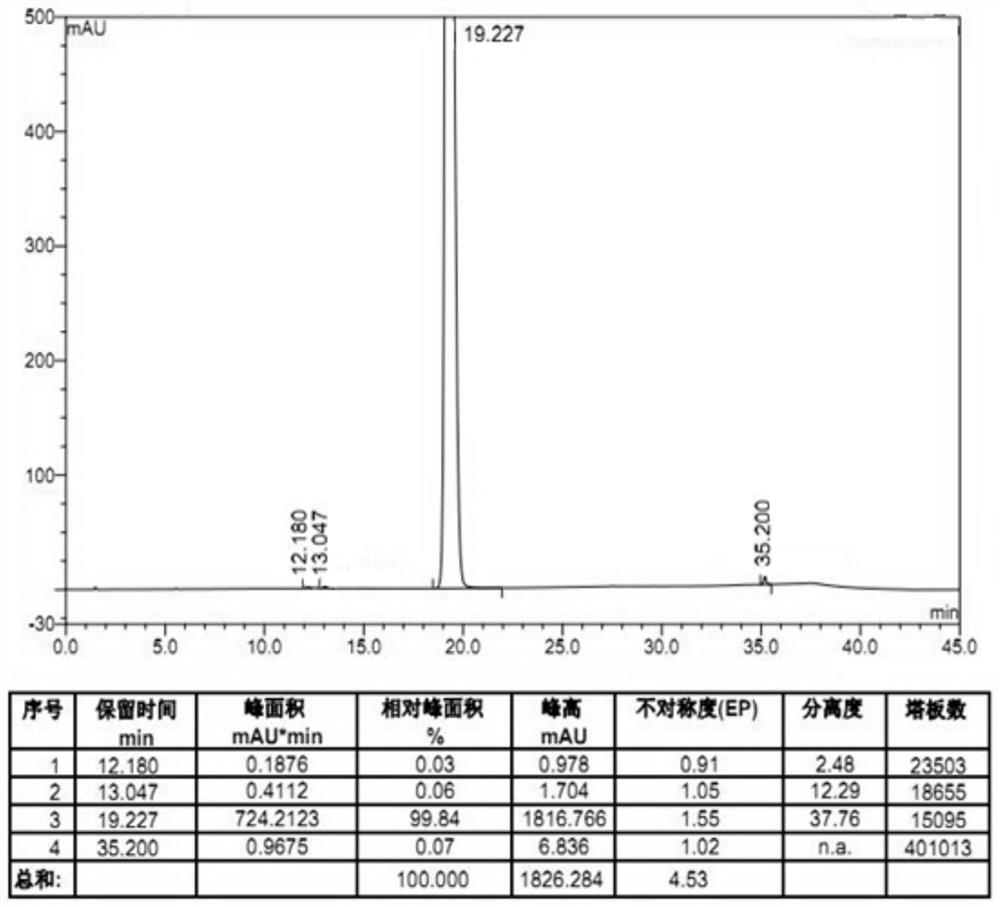
[0037] With 27.5g 4-amino-2-methoxy-5-ethylsulfonylbenzoic acid methyl ester, 39.0g N-ethyl-2-aminomethylpyrrolidine, 50.0g concentration is 20% wt sodium ethylate ethanol solution Add it into 275ml of ethanol, after stirring evenly, raise the temperature to 75°C and keep it warm for 24 hours. After the reaction is completed, concentrate the reaction solution until the solvent no longer drips out, then add 140ml of water to the concentrated reaction solution. Stir at 20°C for 4h, a white solid precipitates, filter, add 140ml of water to the obtained white solid for beating for 1h, filter, wash, and blow dry at 50°C for 15h to obtain a white solid, amisulpride 35.5 g, HPLC purity 99.84%, yield 95.6%, residual methyl amiate 0.06%, other impurities total 0.10%.
Embodiment 3
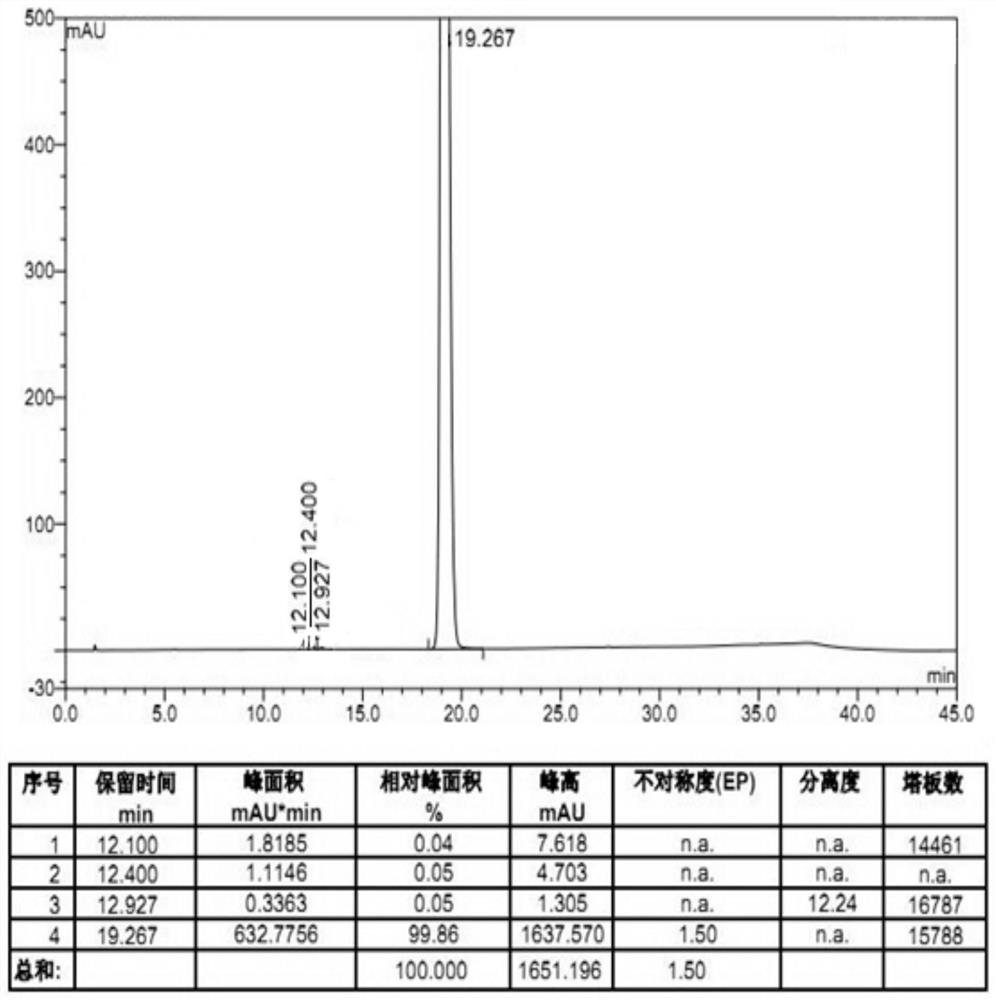
[0039] Add 136.6g methyl 4-amino-2-methoxy-5-ethylsulfonylbenzoate, 64.2g N-ethyl-2-aminomethylpyrrolidine, 96.1g sodium tert-butoxide to 956.2ml tert In butanol, after stirring evenly, raise the temperature to 83°C and keep it warm for 18 hours. After the reaction is completed, concentrate the reaction solution until the solvent no longer drips out, then add 820ml of water to the concentrated reaction solution, and heat it at 10-20°C Stirred under the conditions of 4h, white solid precipitated, filtered, 820ml of water was added to the obtained white solid for beating for 1h, filtered, washed, and air-dried at 50°C for 15h to obtain 172.6g of white solid amisulpride, The HPLC purity is 99.86%, the yield is 93.7%, the residue of methyl amiate is 0.05%, and the total amount of other impurities is 0.09%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com