Preparation method for pharmacopoeia impurity of amisulpride
A technology of amisulpride and Pharmacopoeia, which is applied in the field of medicinal chemistry and can solve problems such as odor and odor
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
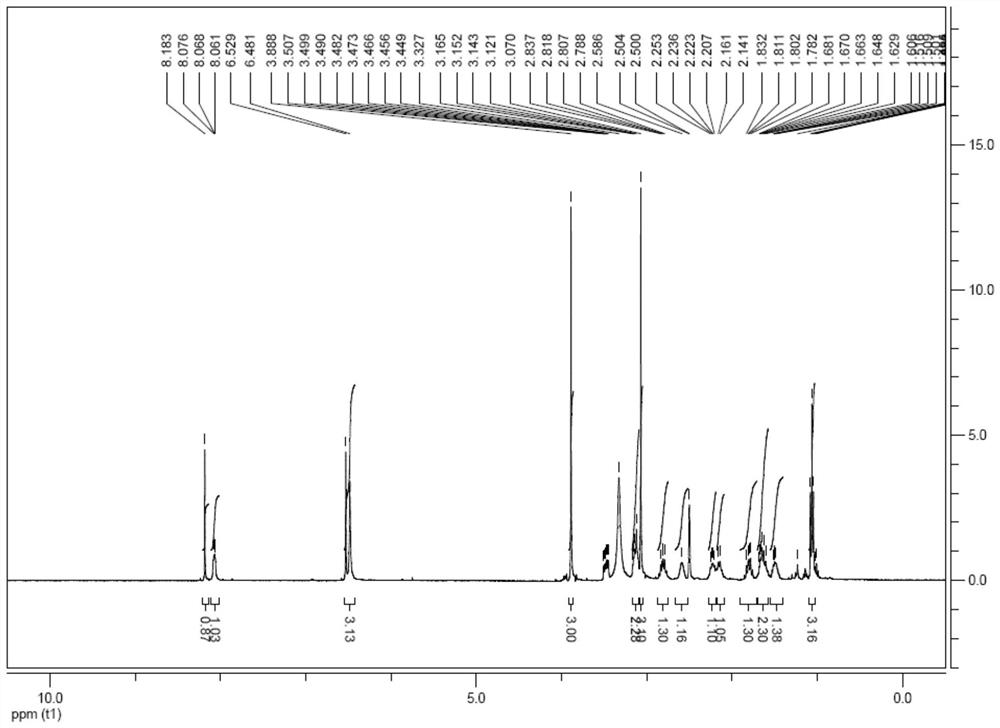
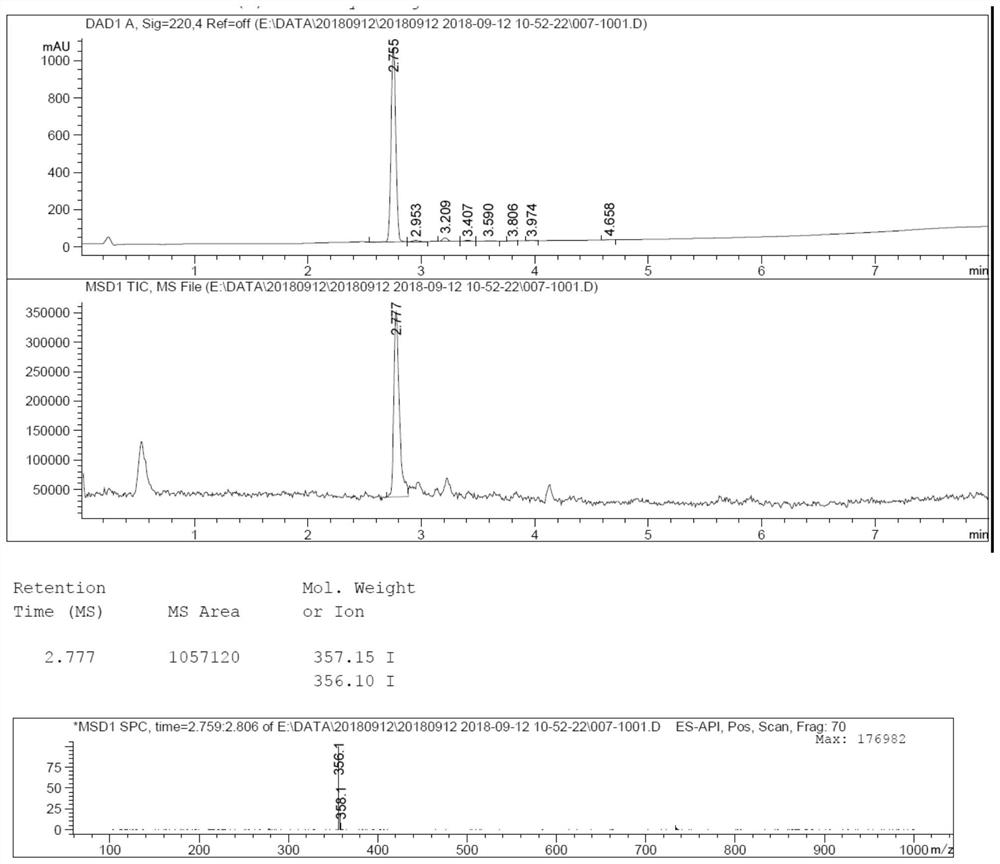
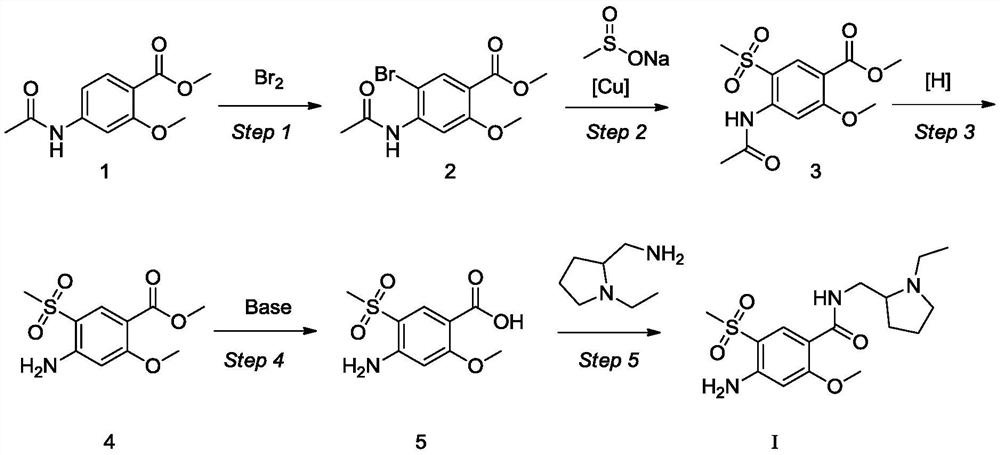
[0024] Example 1: Control the temperature at 15-30°C, add 20.0g of compound 1 into 70mL of acetic acid, slowly add 15.3g of liquid bromine to the reaction, a large amount of solids precipitate, stir for 5-6 hours, slowly drop Add 50mL of n-heptane to the reaction, stir for 1-2 hours, filter, rinse the filter cake with n-heptane, then dissolve the filter cake in 300mL of dichloromethane and 50mL of water, stir and separate the liquid, and adjust the organic phase with saturated sodium carbonate solution pH = 8, stirred and separated, collected the organic phase of dioxymethane, concentrated the organic phase of ethyl acetate under reduced pressure until no liquid flowed out, added 50mL of methyl tert-butyl ether, stirred for 1-2 hours, filtered, methyl tert-butyl The filter cake was rinsed with ether, and the wet product was vacuum-dried at 60° C. to obtain 25.7 g of compound 2 as a yellow solid, with a yield of 95%.
Embodiment 2
[0025] Example 2: Control the temperature at 15-30°C, add 5.0g of compound 1 into 17.5mL of methanol, slowly add 3.8g of liquid bromine dropwise to the reaction, a large amount of solids precipitate, stir for 5-6 hours, slowly Add 12.5mL of n-heptane dropwise to the reaction, stir for 1-2 hours, filter, rinse the filter cake with n-heptane, then dissolve the filter cake in 75mL of dichloromethane and 12.5mL of water, stir and separate the liquids, and then refill the organic phase with saturated carbonic acid Adjust pH=8 with sodium solution, stir and separate the liquids, collect the organic phase of dioxymethane, concentrate the organic phase of ethyl acetate under reduced pressure until no liquid flows out, add 12.5 mL of methyl tert-butyl ether, stir for 1-2 hours, filter, form The filter cake was rinsed with tert-butyl ether, and the wet product was vacuum-dried at 60°C to obtain 5.9 g of compound 2 as a yellow solid, with a yield of 90%.
Embodiment 3
[0026] Example 3: Under the protection of nitrogen, 14.0 g of compound 2 and 23.65 g of sodium methanesulfinate were added to 70 mL of dimethyl sulfoxide, and the air was replaced by nitrogen three times. Under the protection of nitrogen, 44 g of cuprous iodide was added, and the temperature was raised to 90-95°C, stir for 12-16 hours, then cool down to 15-30°C. Add 100mL water to quench the reaction, precipitate solid, filter, rinse the filter cake with 200mL saturated ammonium chloride solution and 15mL ammonia water mixed solution, add the wet product to 100mL saturated ammonium chloride solution and 15mL ammonia water mixed solution, stir for 1-2 hour, filtered, and the filter cake was rinsed with water, and the wet product was vacuum-dried at 60° C. to obtain 11.2 g of compound 2 as a yellow solid, with a yield of 80%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com