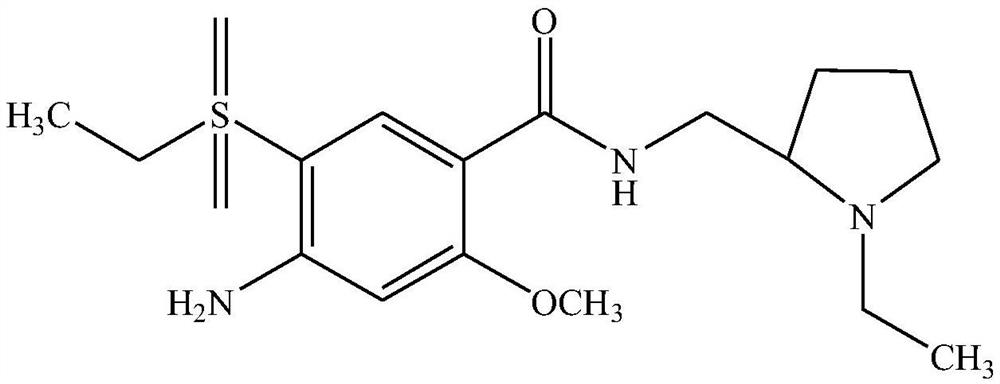
Amisulpride injection
A technology of amisulpride and injection, applied in the field of medicine, can solve problems such as reducing the content of finished products, long stirring time, and unqualified products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1 The effect of different preparation temperatures on the dissolution time of amisulpride.
[0026] Amisulpride injection was prepared with the following formula:
[0027] ingredients Content per ml Amisulpride 2.5mg citric acid monohydrate 9.35mg Sodium citrate dihydrate 16.32mg Sodium chloride 1.8mg Hydrochloric acid / sodium hydroxide Adjust pH to 4.0-7.0
[0028] Preparation method 1:
[0029] (1) at room temperature, in 1L purified water, add citric acid monohydrate, sodium citrate dihydrate and sodium chloride of the formula amount, and stir until completely dissolved;
[0030] (2) adding amisulpride in the solution of step (1) and stirring, the rotating speed is 60r / min, record the time from adding the bulk drug to the complete dissolution of the bulk drug, and the rotating speed is 60r / min;
[0031] (3) use hydrochloric acid / sodium hydroxide in the solution of step (2), adjust pH to 6.0;
[0032] (4) Use...
Embodiment 2
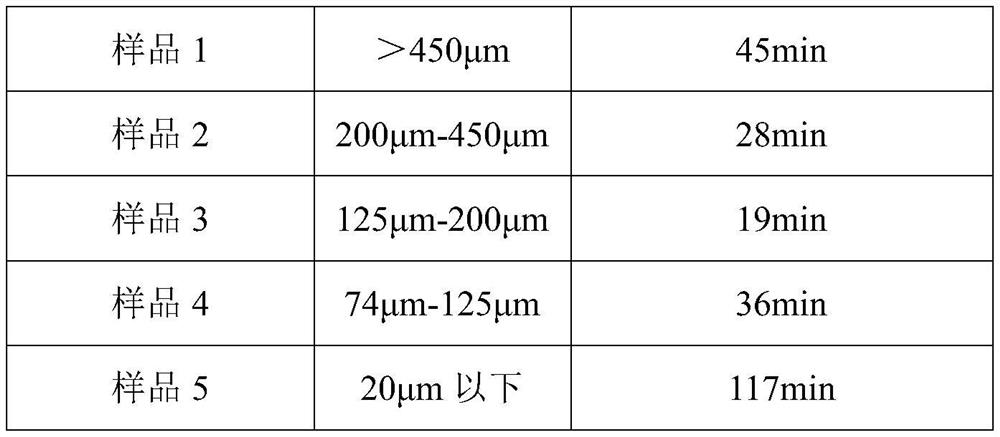
[0040] Example 2 The influence of the particle size of the bulk drug on the dissolution time of amisulpride
[0041] Raw material drug recrystallization: add amisulpride into isopropanol, heat and dissolve to make a saturated solution, filter and cool it at 2-8℃ until crystals separate out, filter and dry at 50℃ to obtain the recrystallized amisulpride raw material .
[0042] The amisulpride raw material of the above-mentioned recrystallization was passed through a 40-mesh sieve (450 μm), and the amisulpride that did not pass through the 40-mesh sieve was sample 1, and its particle size was greater than 450 μm; Pass through an 80-mesh sieve (200 μm) to obtain amisulpride with a particle size between 200 μm and 450 μm as sample 2; continue to pass the sample that has passed through an 80-mesh sieve (200 μm) through a 120-mesh sieve (125 μm) to obtain a particle size of 125 μm- Amisulpride between 200 μm is sample 3; the sample passed through a 120-mesh sieve (125 μm) continues...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com