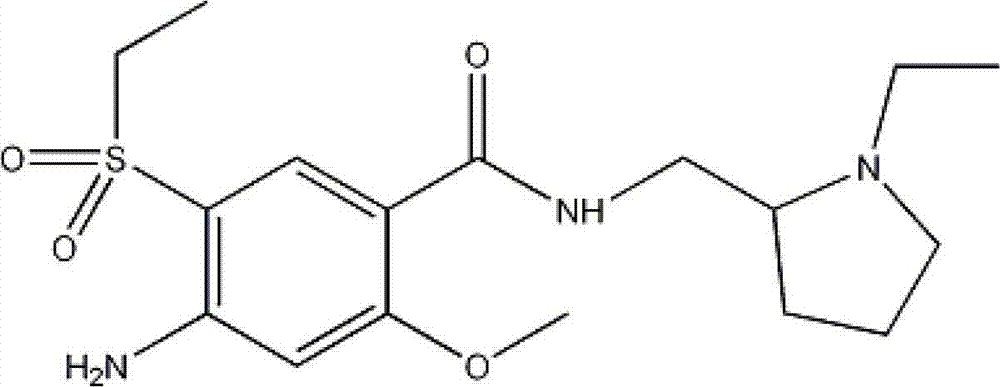
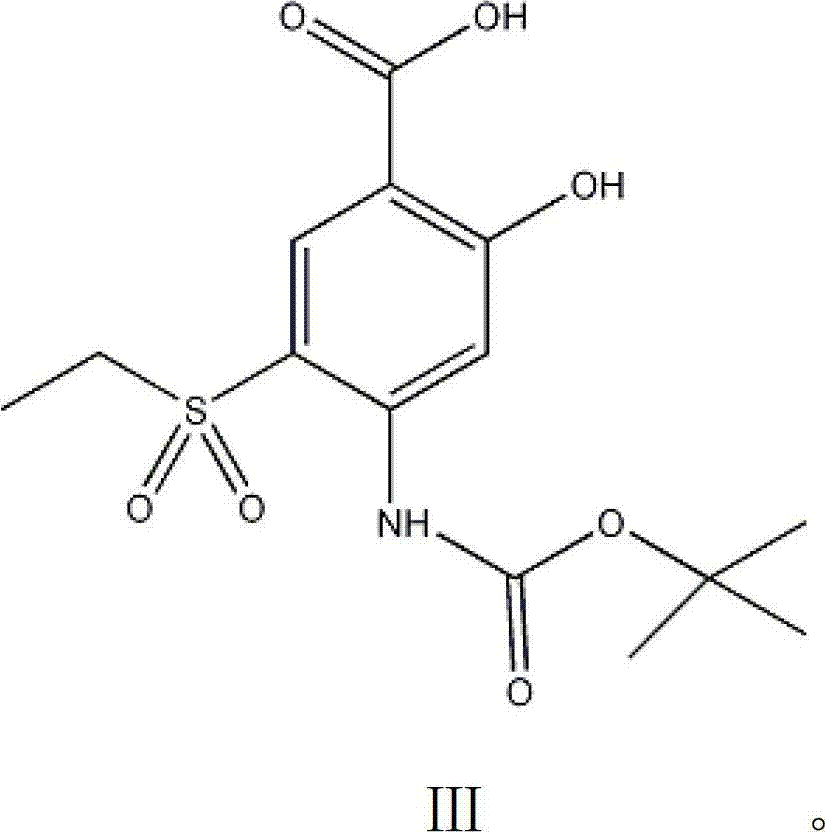
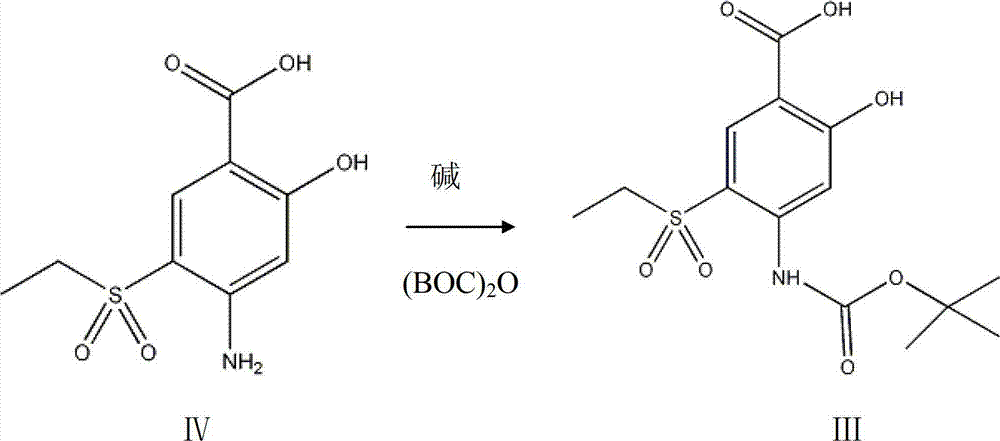
Intermediate in amisulpride and method for preparing amisulpride by using intermediate
A technology of amisulpride and intermediates, applied in the field of preparation of amisulpride, can solve problems such as difficult to remove, reduce yield, affect product quality, etc., and achieve the effect of avoiding impurities and improving quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Intermediate of amisulpride: Synthesis of 4-(N-tert-butoxycarbonyl)amino-2-methoxy-5-ethylsulfonylbenzoic acid:
[0034] After stirring and dissolving 26g of 4-amino-2-methoxy-5-ethylsulfonylbenzoic acid (IV) and 150ml of ethanol, add 12g of sodium carbonate, add 30g of di-tert-butyl dicarbonate at 20°C, and heat to React at 30°C for 5 hours, cool the reaction solution to room temperature, then pour into ice water to separate layers, adjust the pH value of the water layer to 3-4 with dilute hydrochloric acid, stir to precipitate a solid, filter and wash the filter cake with a small amount of distilled water, and dissolve the obtained solid at 40 Drying under reduced pressure at °C yielded 31 g of 4-(N-tert-butoxycarbonyl)amino-2-methoxy-5-ethylsulfonylbenzoic acid (III).
Embodiment 2
[0036] Synthesis of amisulpride: 31g of 4-(N-tert-butoxycarbonyl)amino-2-methoxy-5-ethylsulfonylbenzoic acid was mixed with 12g of N-ethyl-2-aminomethylpyrrolidine Add 100ml of dichloromethane and stir to dissolve, add 19.5g of DCC and stir at 30°C for 12 hours, wash the reaction solution with ice water, distill off the dichloromethane to obtain 44g of 4-(N-tert-butoxycarbonyl) Amino-N-((1-ethyl-2-pyrrolidinyl)methyl)-5-ethylsulfonyl-2-methoxybenzamide; Add 50ml of ethanol and 5ml of 2M hydrochloric acid to stir at room temperature Remove the BOC protecting group in 30 minutes, remove ethanol by distillation under reduced pressure, add 100ml distilled water and stir in the residue, separate out the solid, filter, and recrystallize with acetone to obtain 29.3g amisulpride, the yield is 74.8%, and the purity is 99.6 %.
Embodiment 3
[0038] Intermediate of amisulpride: Synthesis of 4-(N-tert-butoxycarbonyl)amino-2-methoxy-5-ethylsulfonylbenzoic acid:
[0039] Stir and dissolve 26g of 4-amino-2-methoxy-5-ethylsulfonylbenzoic acid (IV) and 150ml of DMF, add 12g of sodium carbonate, add 28g of di-tert-butyl dicarbonate at 20°C, and heat to 60°C React for 0.5 hours, cool the reaction solution to room temperature, then pour into ice water to separate layers, adjust the pH value of the water layer to 3-4 with dilute hydrochloric acid, stir to precipitate a solid, filter and wash the filter cake with a small amount of distilled water, and store the obtained solid at 45°C Drying under reduced pressure gave 31.4 g of 4-(N-tert-butoxycarbonyl)amino-2-methoxy-5-ethylsulfonylbenzoic acid (III).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com