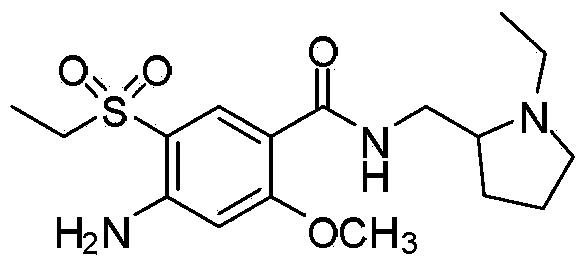
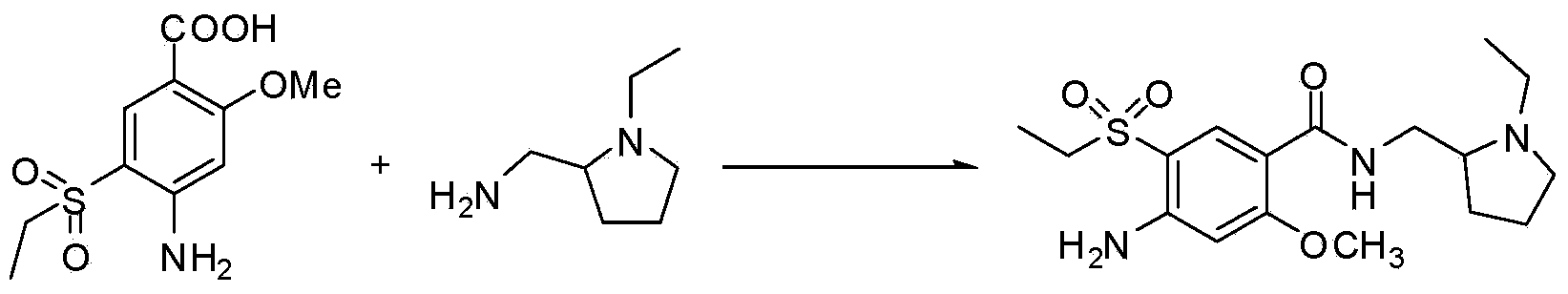
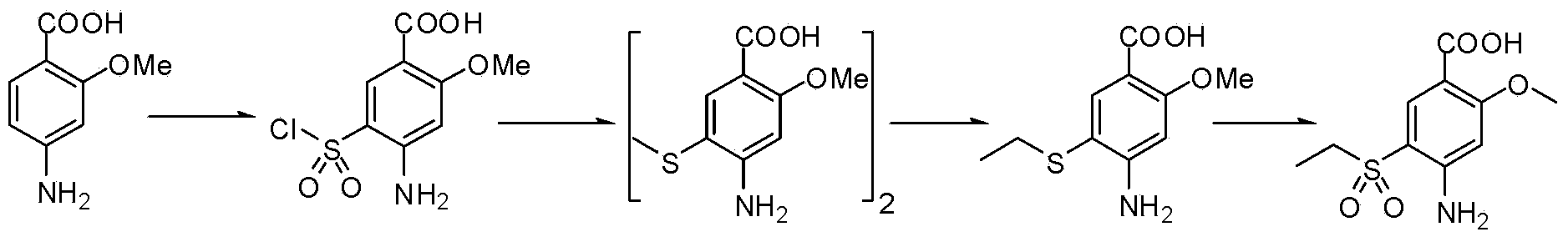
Method or preparing amisulpride acid
Amisulpride acid and amino technology, applied in the field of medicine and chemical industry, can solve the problems of strong acidity, high equipment requirements, unfavorable workshop material feeding, etc., and achieve the effects of low cost, low equipment requirements, and small residual solvent content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] (1) Mix 140g of 4-aminosalicylic acid, 1640g of acetone, and 7g of tap water, add 129g of potassium hydroxide at 20-30°C, and then add 250g of dimethyl sulfate dropwise. After 2 hours of reaction at 200 rpm, the acetone was concentrated at 40-50°C, and 1400g of water was added for crystallization for 2 hours. After filtration, 149g of the product, methyl 2-methoxy-4-aminobenzoate, was obtained, with a yield of 90.0%.
[0042] (2) After mixing 44g of methyl 2-methoxy-4-aminobenzoate, 660g of methanol, and 59.5g of potassium thiocyanate, add 50g of bromine dropwise at 0-5°C, and react at 10-20°C after the addition is complete After 4 hours, 440 g of water was added for crystallization for 1 hour, and 53 g of methyl 4-amino-2-methoxy-5-cyanothiobenzoate were obtained by filtration, with a yield of 91.4%.
[0043] (3) Mix 130g of methyl 4-amino-2-methoxy-5-cyanothiobenzoate with 650g of ethanol and 156g of sodium sulfide, heat up to 50-60°C to dissolve, then add 71.5g of br...
Embodiment 2
[0046] (1) Mix 140g of 4-aminosalicylic acid, 1640g of acetone, and 7g of tap water, add 129g of potassium hydroxide at 20-30°C, and then add 250g of dimethyl sulfate dropwise. After 2 hours of reaction at 200 rpm, the acetone was concentrated at 40-50°C, and 1400g of water was added for crystallization for 2 hours. After filtration, 149g of the product, methyl 2-methoxy-4-aminobenzoate, was obtained, with a yield of 90.0%.
[0047] (2) After mixing 44g of methyl 2-methoxy-4-aminobenzoate, 660g of methanol, and 59.5g of potassium thiocyanate, add 50g of bromine dropwise at 0-5°C, and react at 10-20°C after the addition is complete After 4 hours, 440 g of water was added for crystallization for 1 hour, and 53 g of methyl 4-amino-2-methoxy-5-cyanothiobenzoate were obtained by filtration, with a yield of 91.4%.
[0048] (3) Mix 65g of methyl 2-methoxy-4-aminobenzoate with 325g of ethanol and 78g of sodium sulfide, heat up to 50-60°C to dissolve, then add 50g of diethyl sulfate dr...
Embodiment 3
[0051] (1) Mix 140g of 4-aminosalicylic acid, 1640g of acetone, and 7g of tap water, add 129g of potassium hydroxide at 20-30°C, and then add 250g of dimethyl sulfate dropwise. After 2 hours of reaction at 200 rpm, the acetone was concentrated at 40-50°C, and 1400g of water was added for crystallization for 2 hours. After filtration, 149g of the product, methyl 2-methoxy-4-aminobenzoate, was obtained, with a yield of 90.0%.
[0052] (2) After mixing 44g of methyl 2-methoxy-4-aminobenzoate, 660g of methanol, and 59.5g of potassium thiocyanate, add 50g of bromine dropwise at 0-5°C, and react at 10-20°C after the addition is complete After 4 hours, 440 g of water was added for crystallization for 1 hour, and 53 g of methyl 4-amino-2-methoxy-5-cyanothiobenzoate were obtained by filtration, with a yield of 91.4%.
[0053] (3) Mix 130g of methyl 2-methoxy-4-aminobenzoate with 650g of ethanol and 156g of sodium sulfide, heat up to 50-60°C to dissolve, then add 71.5g of bromoethane dr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com