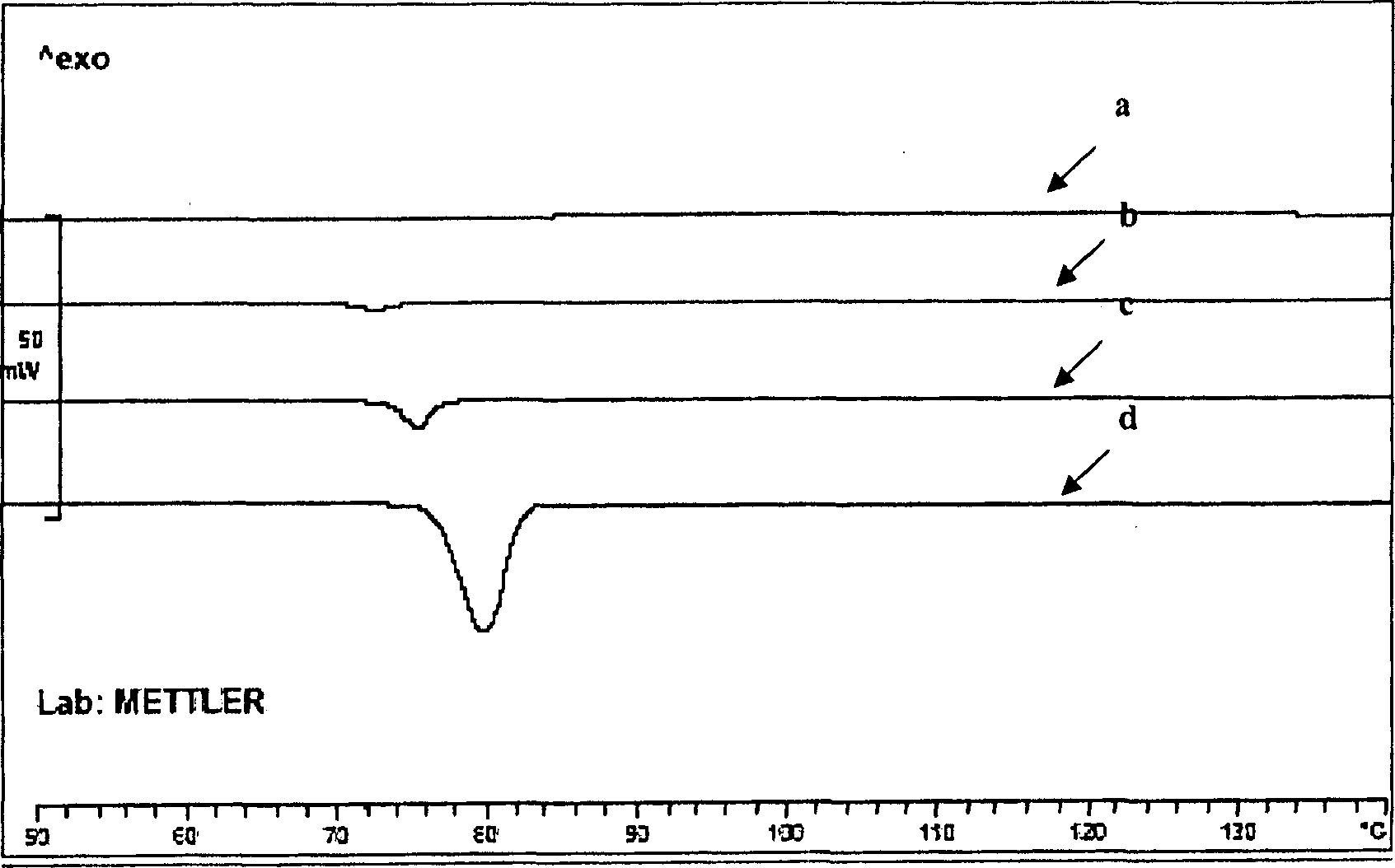
Long acting sustained-release formulation containing dopamine-receptor stimulant medicine and its preparation process
The technology of a receptor agonist and a sustained-release preparation is applied in the field of long-acting sustained-release preparations containing dopamine receptor agonists and their preparation technology, and can solve the problems of no disclosure of composition and ratio.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
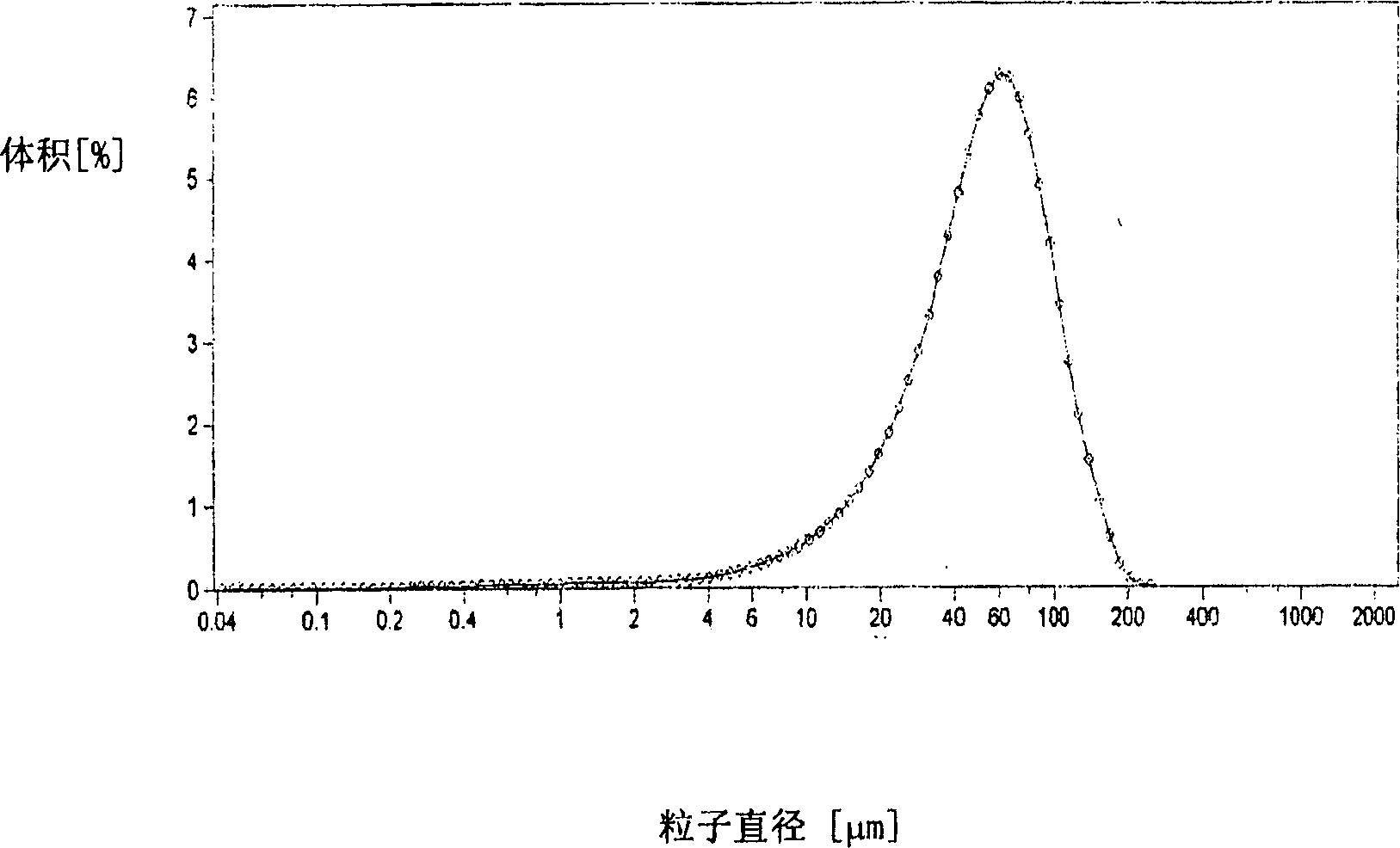
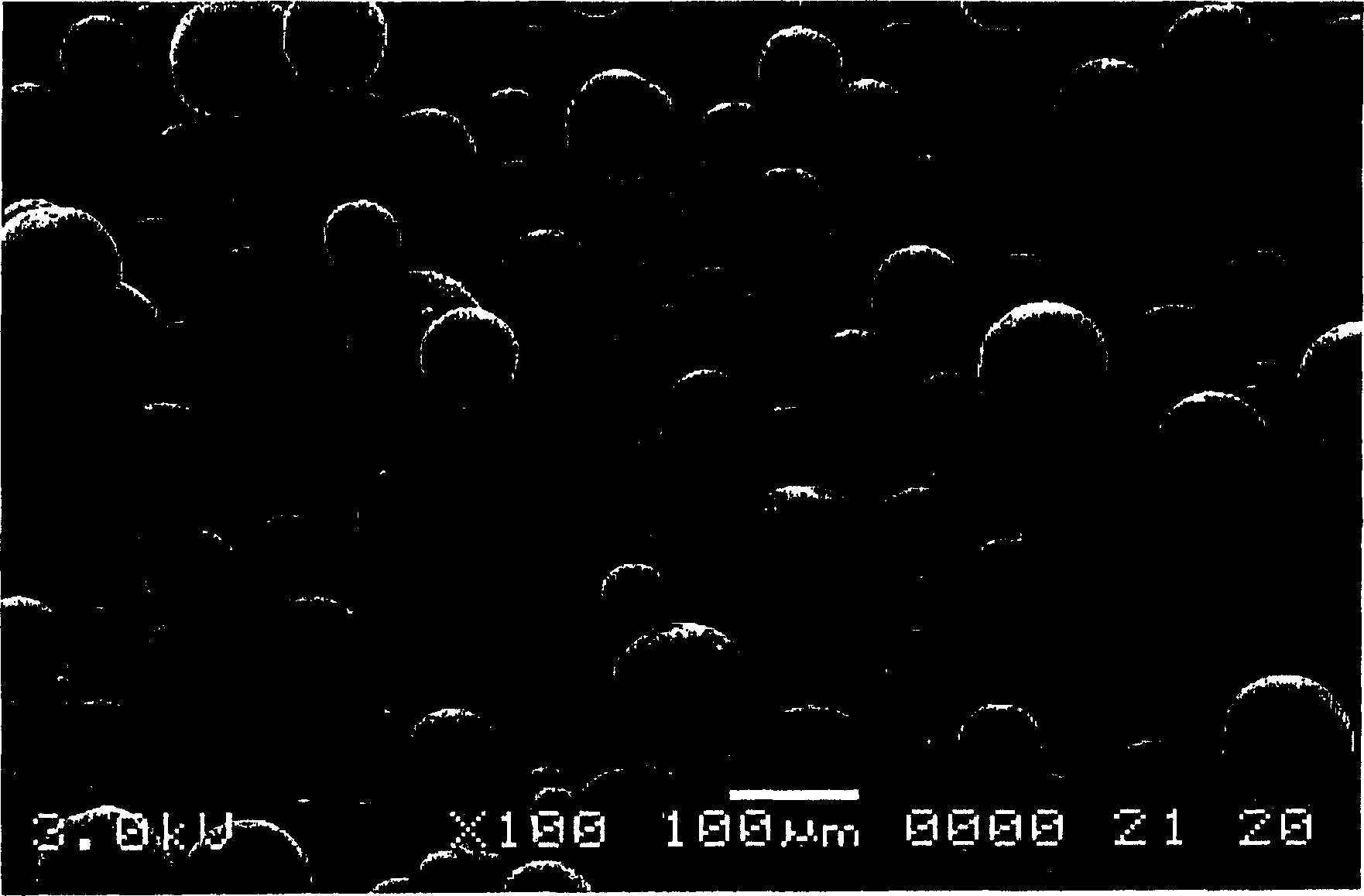
[0127] Dissolve 0.1g of roticotine and 0.9g of polylactide-glycolide (lactide:glycolide=50:50, molecular weight 25,000) in 5ml of dichloromethane, under vigorous stirring (1200-1600rpm) Drop it into 250ml of 0.5% PVA aqueous solution, continue stirring vigorously for 3-10 minutes after dropping, then reduce the stirring speed to 300rpm, volatilize the solvent for 4-6 hours, filter, wash the microspheres with distilled water three times, and freeze-dry. The particle size of the microspheres measured by a laser particle size analyzer is 1-250 μm, and the particle size distribution is as follows: figure 2 shown, and then sieved to remove microspheres with a particle size greater than 150 microns, and subpackaged. image 3 It is the scanning electron micrograph of the slow-release microsphere gained in embodiment 1.
Embodiment 2
[0129] Take by weighing roticotine 0.1g, polylactide-glycolide (lactide: glycolide=50:50, molecular weight 13000) 0.9g, prepare by the method for embodiment 1 and contain medicine 10% particle size is 1-250 μm microspheres. Sieve to remove microspheres with a particle size greater than 150 microns, and divide into packages.
Embodiment 3
[0131] Take by weighing roticotine 0.2g, polylactide-glycolide (lactide: glycolide=50:50, molecular weight 25000) 0.8g, prepare by the method for embodiment 1 containing drug 20%, particle diameter For microspheres of 1-250 μm, but with 250 ml of 0.5% carboxymethylcellulose in water instead of 0.5% PVA in water. Sieve to remove microspheres with a particle size greater than 150 microns, and divide into packages.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com