Respiratory tract delivery of levodopa and dopa decarboxylase inhibitor for treatment of parkinson's disease
A technology of dopa decarboxylase and levodopa, which is applied in the directions of nebulizers for treatment, medical preparations without active ingredients, and medical preparations containing active ingredients, etc., can solve the problems of dysphagia, levodopa, etc. difficulties, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment A
[0165] In one embodiment, a device is constructed and tested. Test the residual powder in the compound container after actuation. When 2 or more but less than 6 grooves are used on the inlet interface, the apparatus has a relatively powder delivery performance, as measured by the actuated residue. In this example, the groove is combined with a 63mg HFA propellant and a nozzle. The aperture is combined. Four grooves (90 degrees) are found to provide a homogeneous gas delivery.
[0166] Dose quality
[0167] Perform dose quality reproducibility test. The standard deviation of the dose delivery indicates that the device can deliver a consistent dose quality. The average dosage residue remaining in the device <5%, indicating that the dose in the device is very small.
[0168]
[0169] Table 0: Quality reproducibility of the final molding device, value in milliga
[0170] 5.5.3.4. Nasal devices with multiple filter
[0171] Figure 9A Another example of the ...
Embodiment 1
[0180] 5.6.1. Example 1: Non-human primate PK research
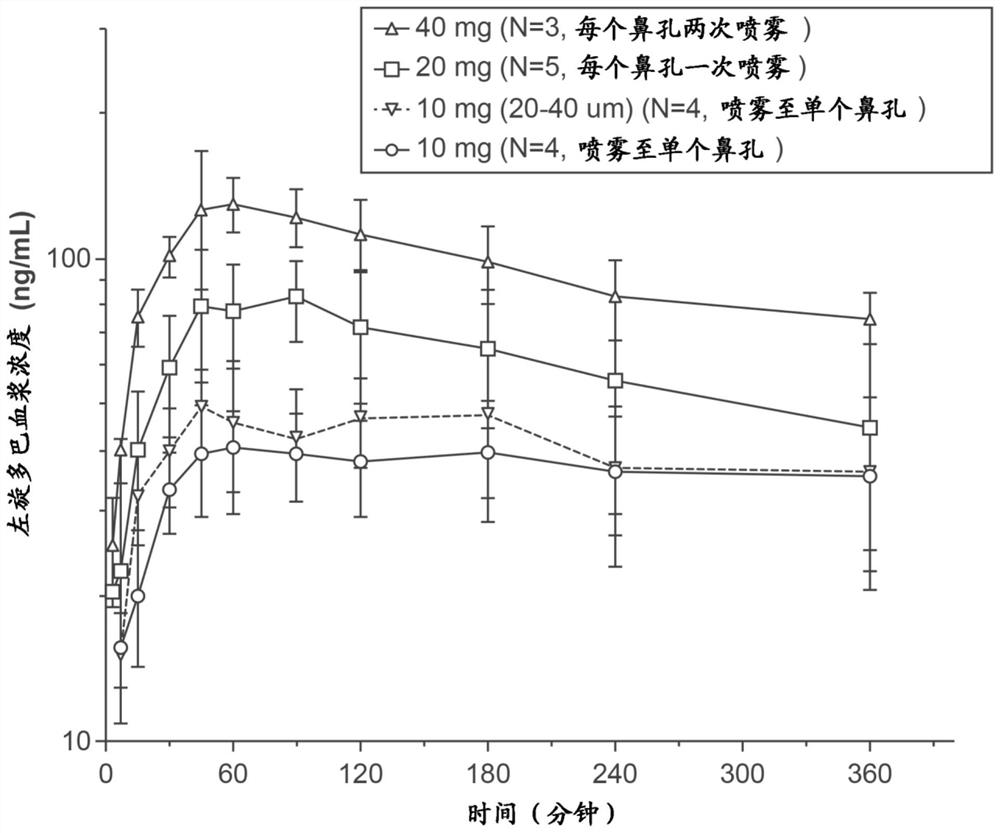
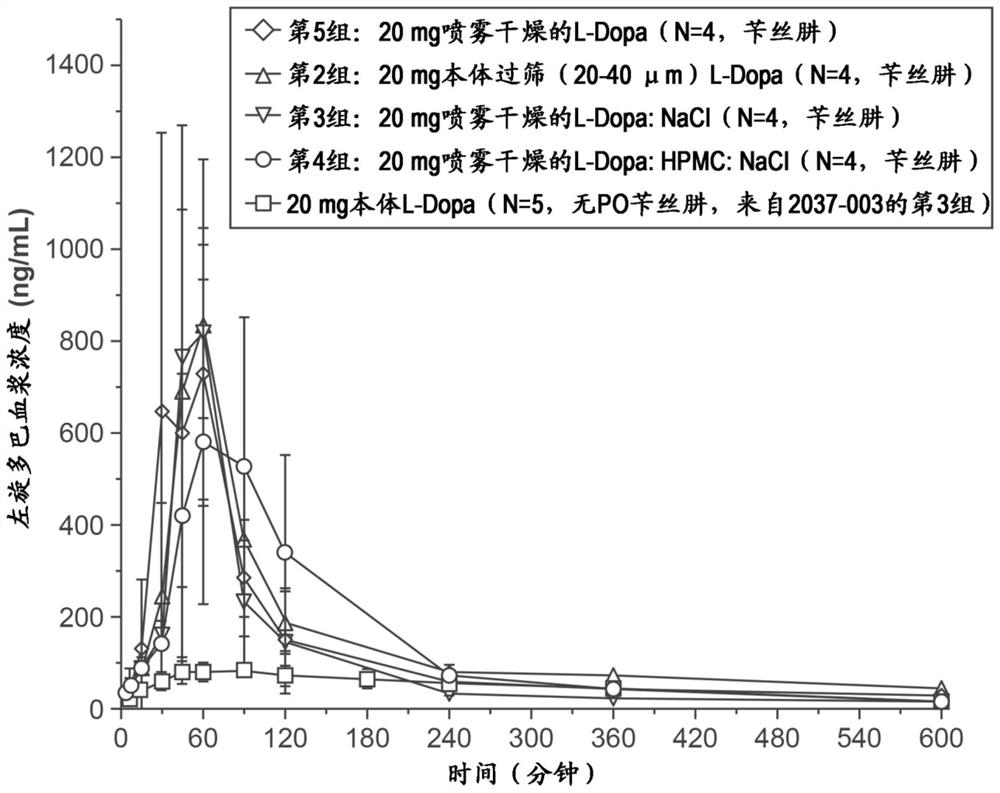
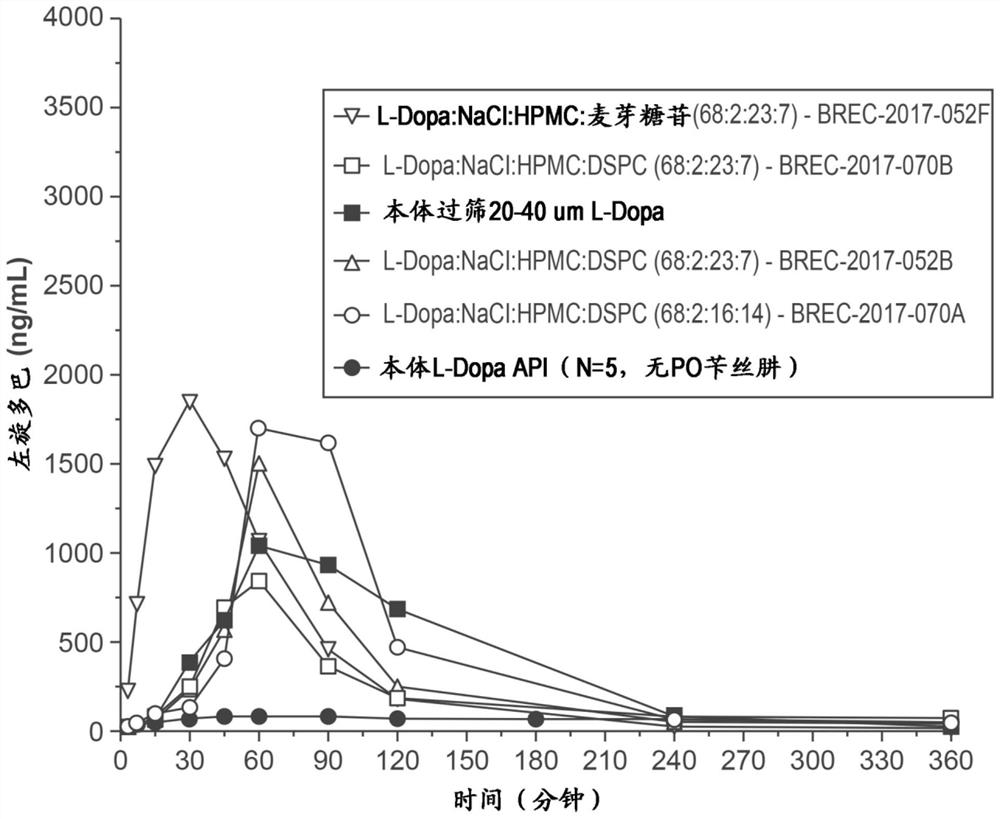
[0181]Developing and manufacturing a series of L-DOPA (L-dopa) powder formulation with or without the DDI (benserazide or carbidopa) levodopa to assess non-human primates ( "NHP" Pharmacokinetics in the Nasal Nasal Division. Formulation development target is to obtain a powder using a nasal delivery precision non-human primate ( "nhpPOD") after intranasal delivery device leads to a rapid increase in plasma concentrations Formulation> 200-400ng / mL of the formulation such that It is expected to actively affect the "OFF" on Parkinson's disease.
[0182] Six single dose PK studies were carried out in the cynomolus monkeys to examine the PK after administration of the poly powder L-DOPA formulation delivered by the NHPPOD device through the intranasal pathway. Check unmodified formulation comprising crystalline powder (median particle size of about 50μm), sieved formulation crystalline L-DOPA-containing particles to determine t...
Embodiment 2
[0306] 5.6.2. Example 2: Rethinking PK Research
[0307] 5.6.2.1. Single dose of intranasal pharmacokinetics in rats (Research No. PBI-18-057)
[0308] Single dose PK studies were carried out in Sprague-Dawley rats (weight of 226-250 g), in which to use or without benzyl hydrazine pretreatment, the intranasal administration ("RPOD") is administered in nasal administration ("RPOD"). DOPA drying powder (spray dry) formulation. The RPOD used in this experiment is an intranasal delivery device for rats, which is described in U.S. Patent Publication No. 2015 / 0100042, which is incorporated herein by reference. Twenty male rats were divided into five groups. According to the description summarized in Table 19, different L-DOPA spray drying preparations were administered each group.
[0309] Pre-administration of each animal in the first group 1-4 was preprocessed using 3 mg / k oral benzyl hydrazine using 3 mg / K orally. Animals in the 5th group do not use oral benzyl hydrazine pretreat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com