Topical composition for follicular delivery of an ornithine decarboxylase inhibitor
A technology of ornithine decarboxylase and composition, which is applied in drug combination, pill delivery, aerosol delivery, etc., and can solve problems such as difficult to distinguish the efficacy of DFMO preparations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
Method to measure drug delivery to hair follicles
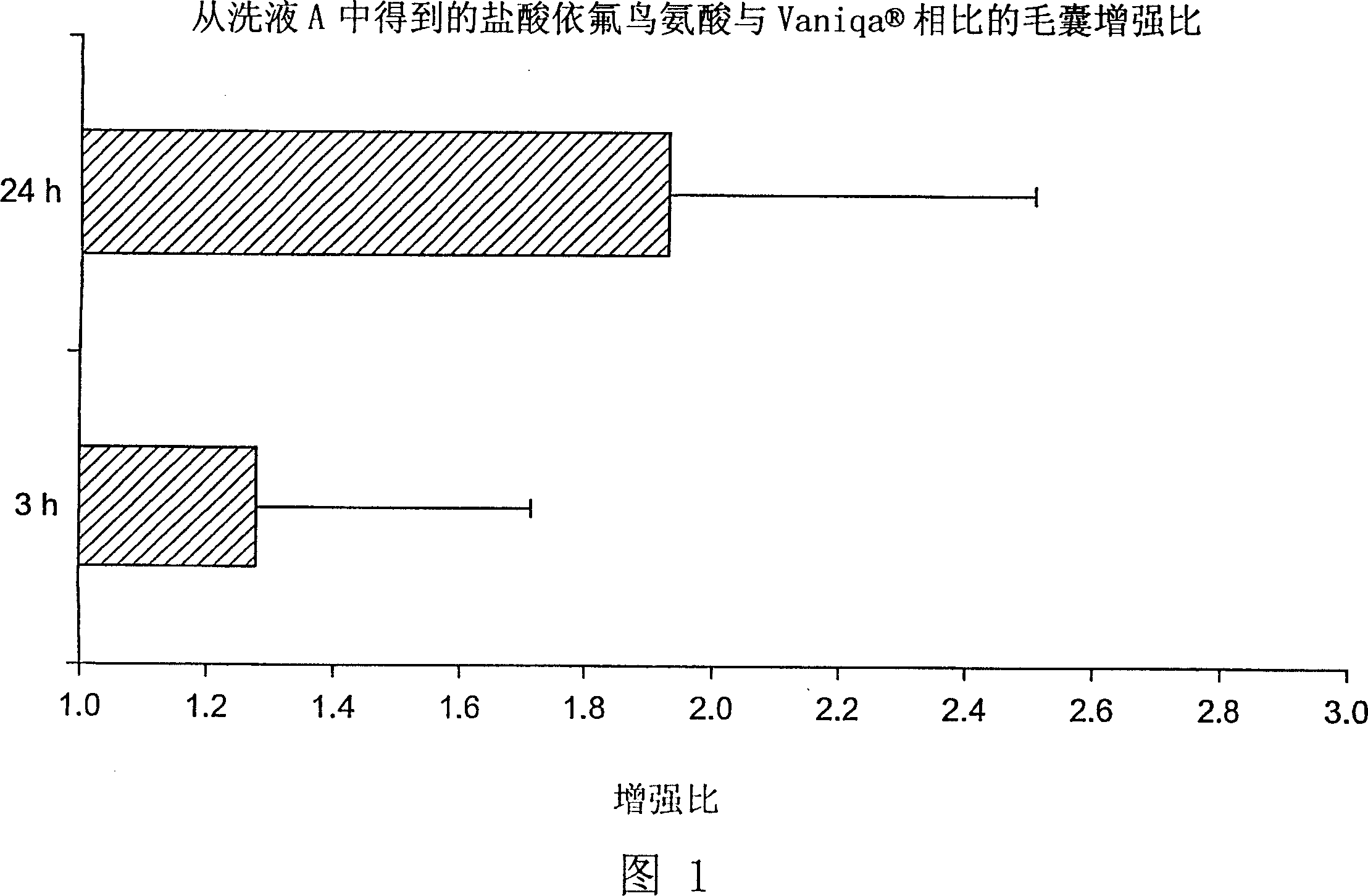
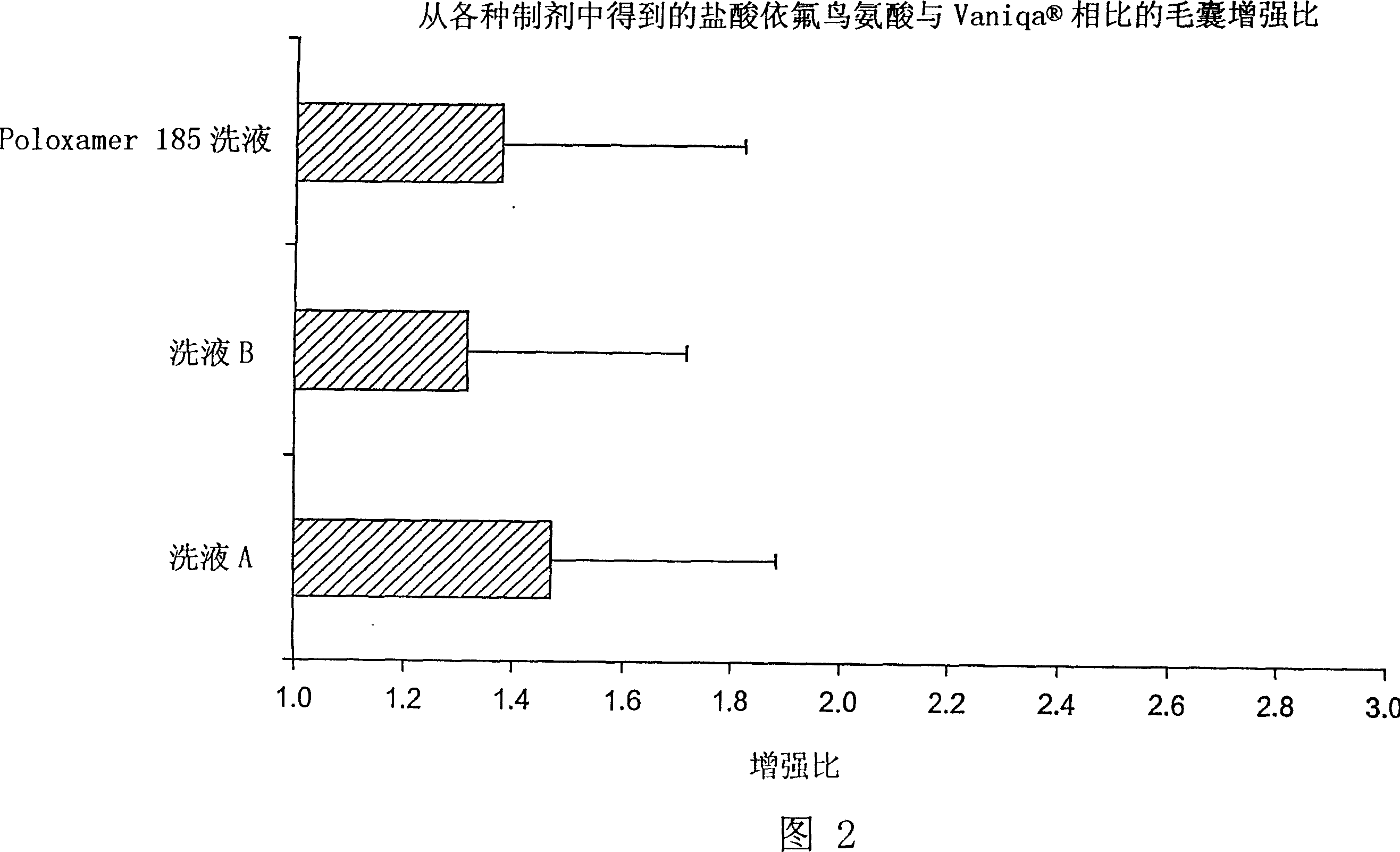
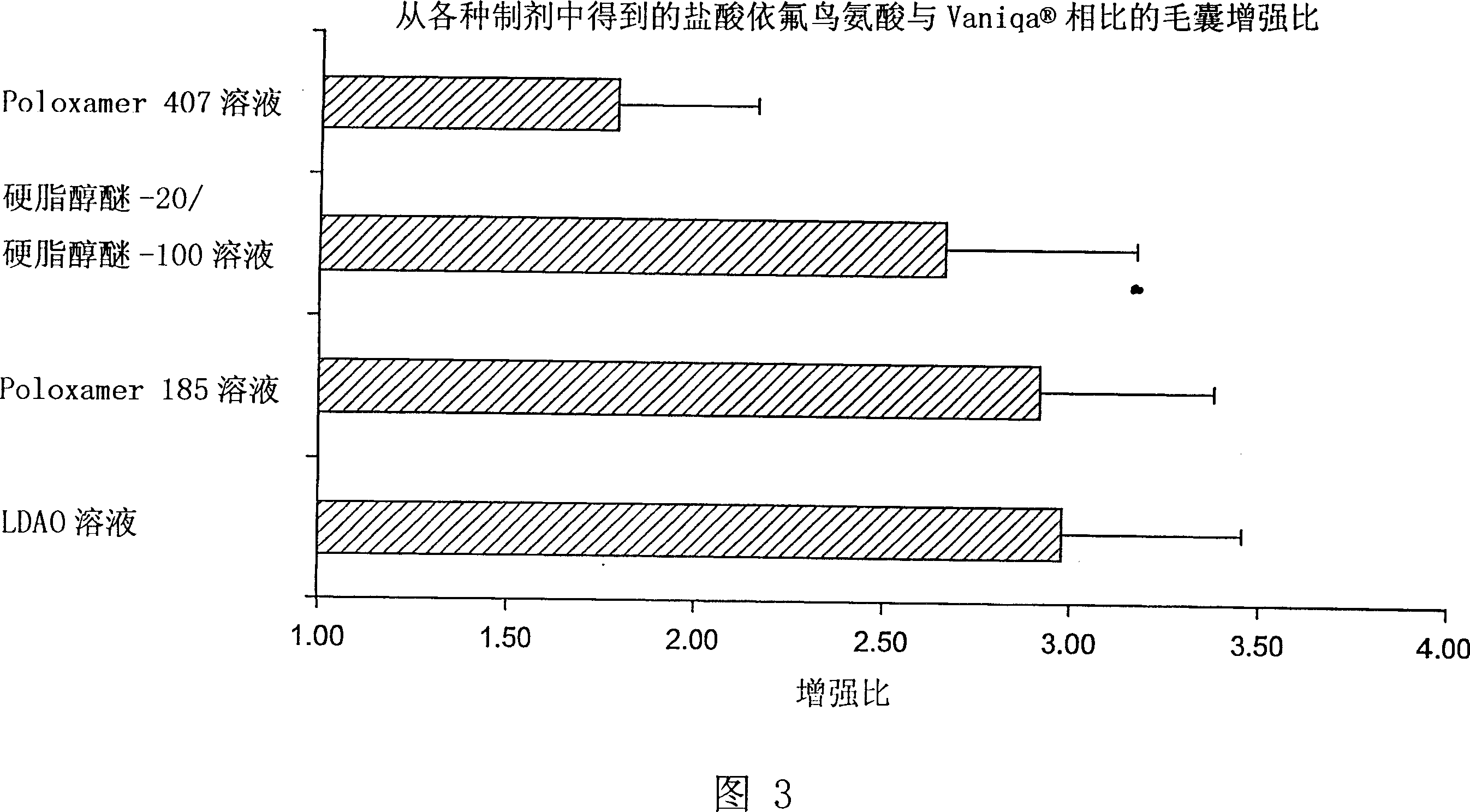
[0040] Dead body scalp or bearded skin with hair was collected within 24 hours of death. The removed skin was stored at -70°C. The refrigerated skin was thawed in normal saline maintained at 30°C before use. After thawing, excess subcutaneous fat was clipped from the skin, and the hair was trimmed using electric scissors so that the hair length on the skin surface was 1-2 mm. The skin was then cut into slices of appropriate size and placed in a Franz diffusion cell (0.63 cm 2 ), maintained at 37°C, as the acceptor phase. 30 [mu]l of radiolabeled preparation (1-3 [mu]Ci of radiolabeled eflornithine hydrochloride, 99% minimum radiochemical purity) was gently applied to the skin surface with a glass rod. In vitro diffusion studies were performed 3 to 24 hours after application of the formulation, with Vaniqa in each study as a positive control. The amount of drug delivered to the hair follicle can be measured by epilati...
Embodiment 2
Lotion A
components %
Stearyl ether-20 5
Stearyl ether-100 5
Mineral oil 1.9
Stearyl Alcohol 3
Simethicone-200 1
Cetyl Alcohol 1
Eflornithine Hydrochloride Monohydrate * 15
water 68.1
* Equivalent to 13.9% eflornithine hydrochloride
[0045] The preparation of above-mentioned lotion composition is as follows:
[0046] The aqueous phase consisting of eflornithine hydrochloride monohydrate and water is heated to 65-70°C. Heat the oil phase consisting of the remaining ingredients to 65-70°C. The oil and water phases were mixed under stirring and the mixing was continued until the temperature was 30-35°C.
Embodiment 3
Lotion B
components %
Stearyl ether-20 5
Stearyl ether-100 5
Mineral oil 1.9
Stearyl Alcohol 3
Simethicone-200 0.5
Glyceryl Stearate and PEG-100 Stearate 2.5
Cetearyl Alcohol and Ceteareth-20 2.5
Eflornithine Hydrochloride Monohydrate * 15
water 64.6
* Equivalent to 13.9% eflornithine hydrochloride
[0047] The above-mentioned lotion composition was prepared according to the method of Example 2.
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com