Dopa decarboxylase inhibitor compositions
A composition, levodopa technology, applied in the field of kits, can solve the problems of invasiveness and inconvenience
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] Embodiment 1. preparation preparation method
[0069] Formulations of levodopa (LD) and carbidopa (CD) can be prepared as follows:
[0070] Method #1 (L-Arg solution): Dissolve L-Arg and sodium bisulfite (Na-Bis) in water. The solution was added to the LD and CD powders. The mixture was stirred and heated at 75°C for 13 minutes until complete dissolution. The LD / CD solution was kept at room temperature (RT) for 10 minutes to cool.
[0071] Method #2 (all powders together): Weigh all powders (LD, CD and L-Arg) and add Na-Bis in water. The suspension was heated with stirring at 75°C for 13 minutes until complete dissolution. The LD / CD solution was kept at RT for 10 min to cool.
[0072] Method #3 (same as #2 without Na-Bis preheat): Weigh all powders (LD, CD and L-Arg) together and add water. The suspension was heated with stirring at 75°C for 13 minutes until complete dissolution. The LD / CD solution was kept at RT for 10 min to cool.
[0073] Method #4 (step prepar...
Embodiment 2
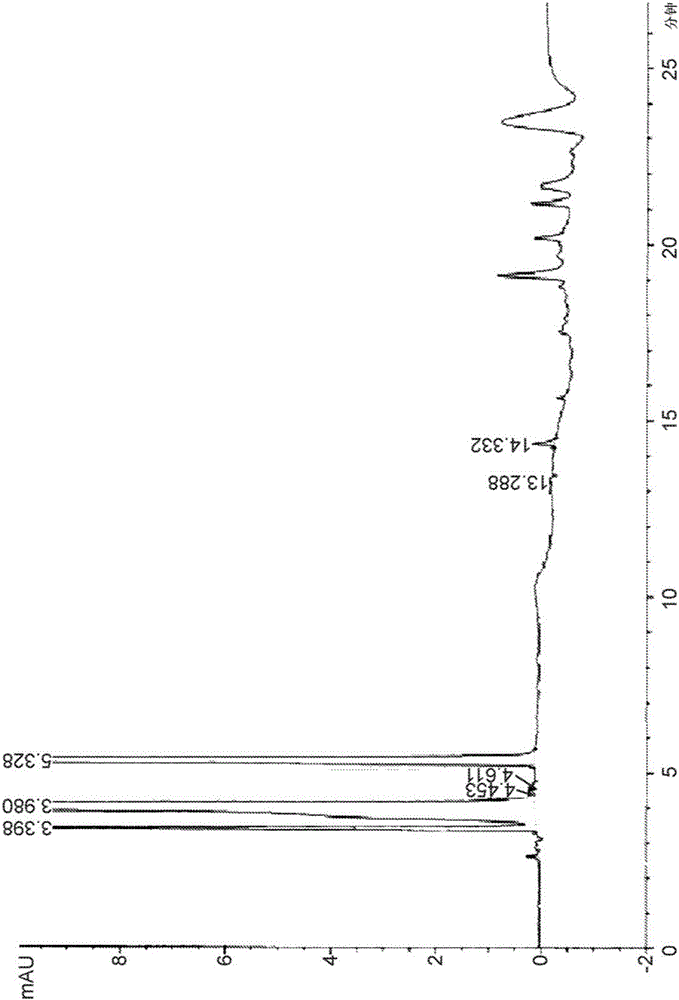
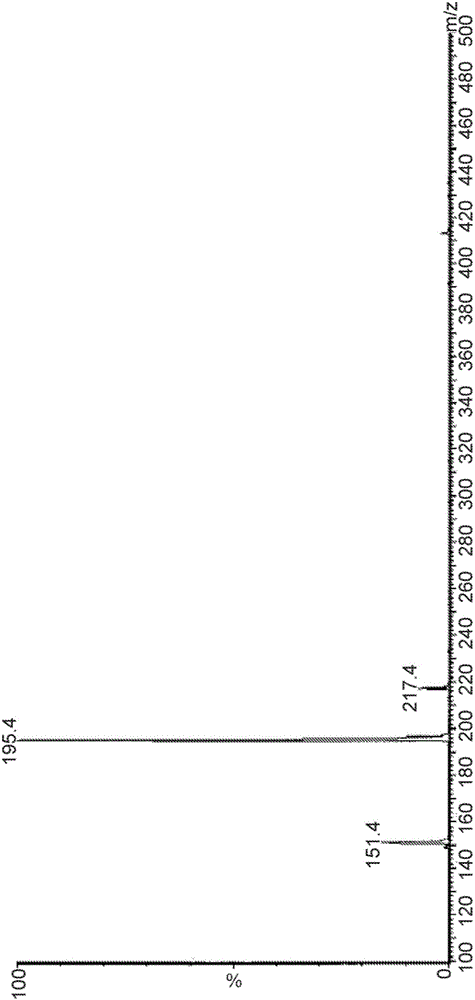
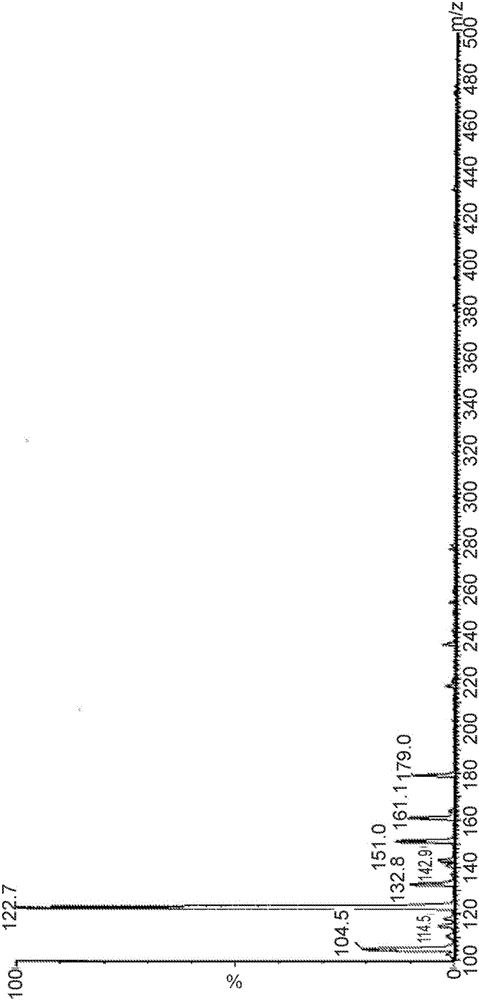
[0076] Example 2. Identification of major degradants in CD-containing formulations
[0077] A liquid formulation with levodopa, carbidopa and arginine was prepared using the method outlined in Example 1, and the carbidopa levodopa formulation was subjected to HPLC analysis using the Agilent 1100 system according to the APH Stability Indicating Analytical Method .
[0078] The HPLC system used here included the following components manufactured by Agilent: pump system (model G1311A), diode array detector (model G1315B), autosampler (model G1329A), degasser (model G1379A), thermostat (model G1330B), thermostat column compartment (model G1316A). The column used is a new Synergi 4μFusion-RP 80A, 250×4.6mm
[0079] HPLC working conditions : wavelength: 280nm; flow rate: 1.0ml / min; injection volume: 10μl; column temperature: 30°C; thermostat temperature: 4°C; stop time: 27 minutes; pressure: 105bar.
[0080] mobile phase preparation : solvent A: acetonitrile, solvent B: 20 ...
Embodiment 3
[0093] Example 3. Effect of ascorbic acid with or without EDTA on the stability of LD / CD formulations
[0094] Liquid formulations were prepared by weighing all powders (LD, CD, EDTA, ascorbic acid and L-Arg) and adding water preheated to 73±3°C. Place the suspension in a water bath at 73±3°C and stir for 10 minutes until completely dissolved. The LD / CD solution was kept at RT for 10 min to cool. The solution was divided into glass vials and kept at +25°C and -20°C for the indicated times. Place frozen vials at RT until completely thawed prior to analysis. The formulations were then mixed and analyzed for stability. The effect of ascorbic acid with or without EDTA on the stability of LD / CD formulations was measured by HPLC. The levels of degradants presented in Table 4 and Table 5 (as a percentage of the initial CD amount) represent the level of stability of the LD / CD formulations and indicate that EDTA has no significant effect on the stability of the LD / CD formulations. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com