Pharmaceutical compositions comprising levodopa, a dopamine decarboxylase inhibitor and a COMT inhibitor and a method of administration thereof
A technology of dopamine decarboxylase and levodopa, applied in drug combination, drug delivery, pharmaceutical formulation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0235] Example 1: Composition
[0236] Hereinafter, the use of the enteral gel composition (hereinafter referred to as "LECIGON" according to the present invention) will be described. TM ”) and commercially available levodopa / carbidopa enteral gels of the prior art (hereinafter referred to as "LCIG"), and the use of modified LECIGON TM Composition for stability testing. LCIG and LECIGON are given in Tables 2 and 3 below TM composition.
[0237] Table 2 Exemplary composition of LCIG
[0238]
[0239] Table 3 LECIGON TM Exemplary composition of
[0240]
[0241] Fifteen exemplary small scale batches as described in Table 4 were prepared as follows and filled into syringes for initial evaluation and stability evaluation under various storage and use conditions. Experimental batch sizes ranged from 100 g to 500 g. In Table 4, API is the active pharmaceutical ingredient, L is levodopa, C is carbidopa, and E is entacapone.
[0242] Table 4
[0243]
[0244]
Embodiment 2
[0245] Embodiment 2: preparation process
[0246] Exemplary preparations of intestinal gel samples to be tested are described below.
[0247] 1) Add sodium carboxymethylcellulose to purified water in a Pyrex beaker and homogenize for 1 to 2 minutes until a viscous solution without lumps is obtained.
[0248] 2) Add the active ingredients levodopa, carbidopa and entacapone and homogenize until a homogeneous suspension is obtained.
[0249] 3) Add additional excipients as described in Table 3 above and homogenize until dissolved.
[0250] 4) If necessary, during mixing, adjust the pH to the target pH by adding sodium hydroxide or hydrochloric acid solution.
[0251] 5) Manually fill the suspension into the syringe.
[0252] The process equipment used to prepare the experimental batches included: Silverson L5M homogenizer (Silverson Machines Ltd., Chesham, U.K.) and IKA Janke & Kunkel RW28W mixer (IKA Works GmbH, Staufen, Germany).
[0253] During preparation, the following...
Embodiment 3
[0257] Embodiment 3: stability test
[0258] This example demonstrates the stability of the enteric gel under various conditions.
[0259] 3.1. Unstable LECIGON TM Stability relative to LCIG
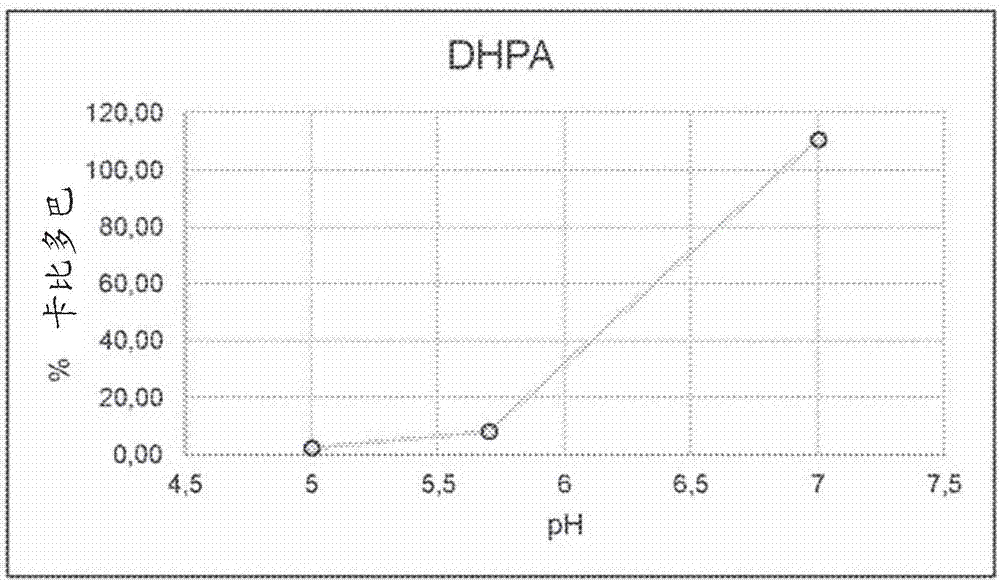
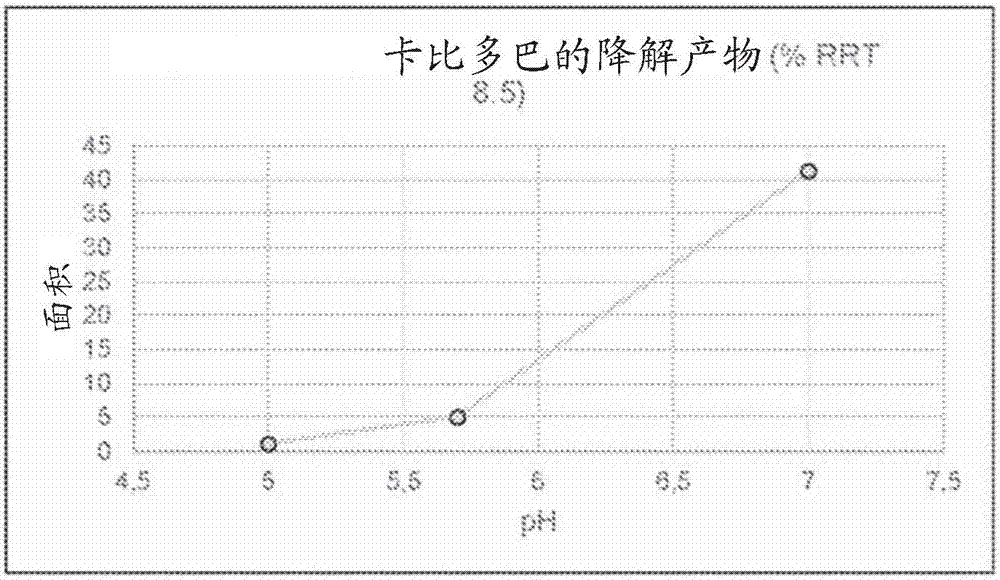
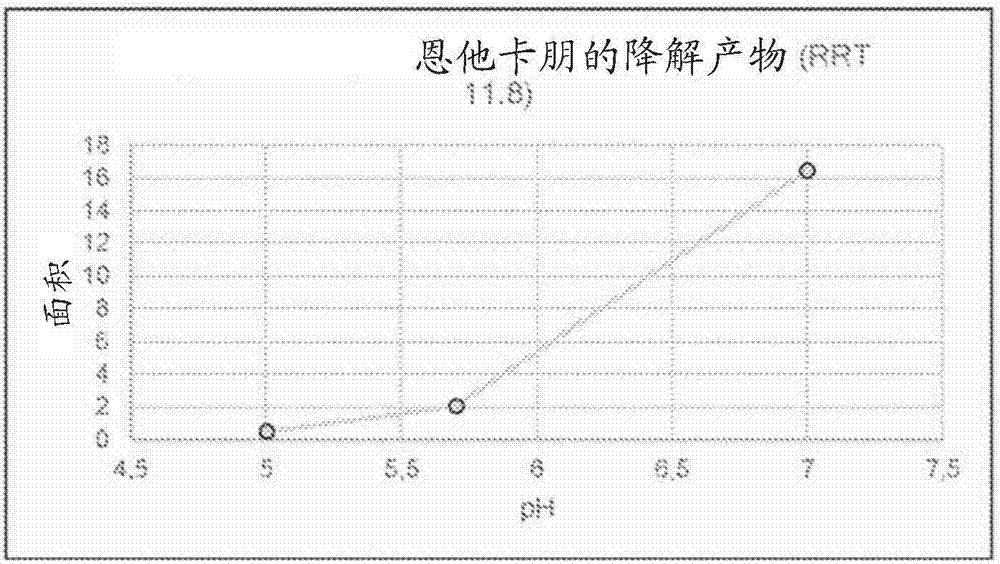
[0260] Unstabilized LECIGON TM The stability of carbidopa, especially the degradation product hydrazine (considered genotoxic) of carbidopa, was compared with that of LCIG. Hydrazine is formed equimolarly with 3,4-dihydroxyphenylacetone (DHPA), which is easier to measure, so in this experiment and others below, DHPA was used as the reference. Compare. The results are shown in Table 5 below.
[0261] table 5.
[0262]
[0263] As shown in Table 5, compared with the corresponding levodopa-carbidopa gel suspension (LCIG), in the three-component levodopa-carbidopa-entacapone gel suspension (LECIGON TM ) degrades about 50% faster in carbidopa.
[0264] 3.2. LECIGON TM stabilization of
[0265] Using LECIGON TM , for carbidopa, levodopa and entacapone, a 12-day stabilization ex...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com