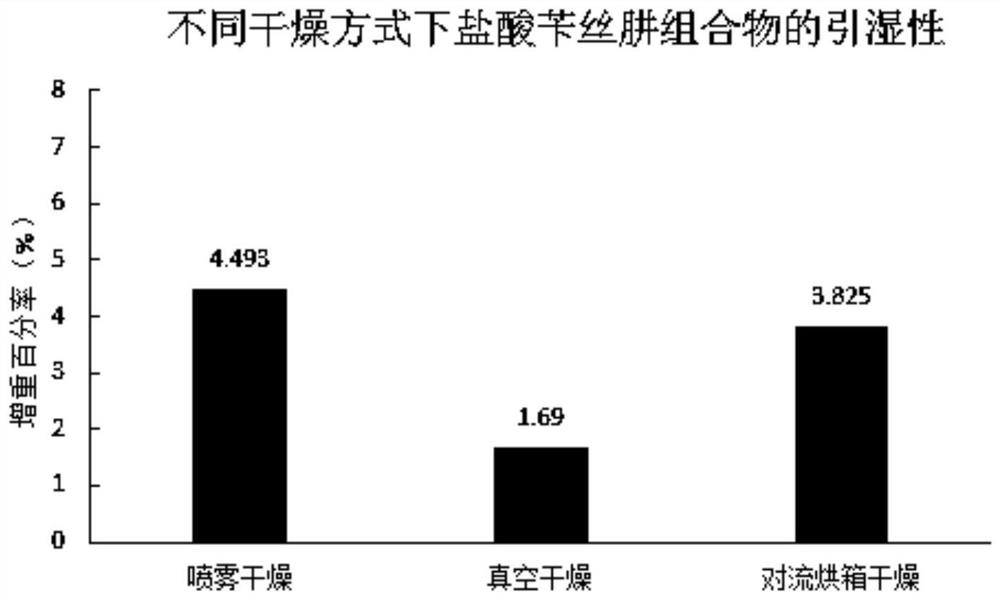
Method for improving stability of benserazide hydrochloride oral administration solid composition
A technology of benserazide hydrochloride and solid composition, which is applied in the field of pharmaceutical preparations, can solve the problems of solid preparation impurity detection exceeding the standard, accelerated long-term test research, and poor bioavailability, etc., to achieve shortened heating time, good protection effect, and complete granules Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-4
[0043] The benserazide hydrochloride-levodopa compound tablet was prepared by wet granulation and vacuum drying.
[0044] prescription composition Prescription mass ratio (%) Levodopa 36.4 Benserazide Hydrochloride 10.2 microcrystalline cellulose 30.8 Mannitol 1.8 Calcium hydrogen phosphate anhydrous 1.8 pregelatinized starch 5 Crospovidone 5 povidone 7 Micropowder silica gel 1 Magnesium stearate 1
[0045] Preparation:
[0046] 1) Weigh the prescription amount of levodopa, microcrystalline cellulose, 2 / 3 prescription amount of crospovidone and mix, make soft material with 5% povidone ethanol solution, granulate with 24 mesh sieve, dry in convection oven for about 2 hours.
[0047] 2) Weigh the prescription amount of benserazide hydrochloride, mannitol, anhydrous calcium hydrogen phosphate, pregelatinized starch, mix with 1 / 3 of the prescription amount of cross-linked povidone, and make soft material wi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com