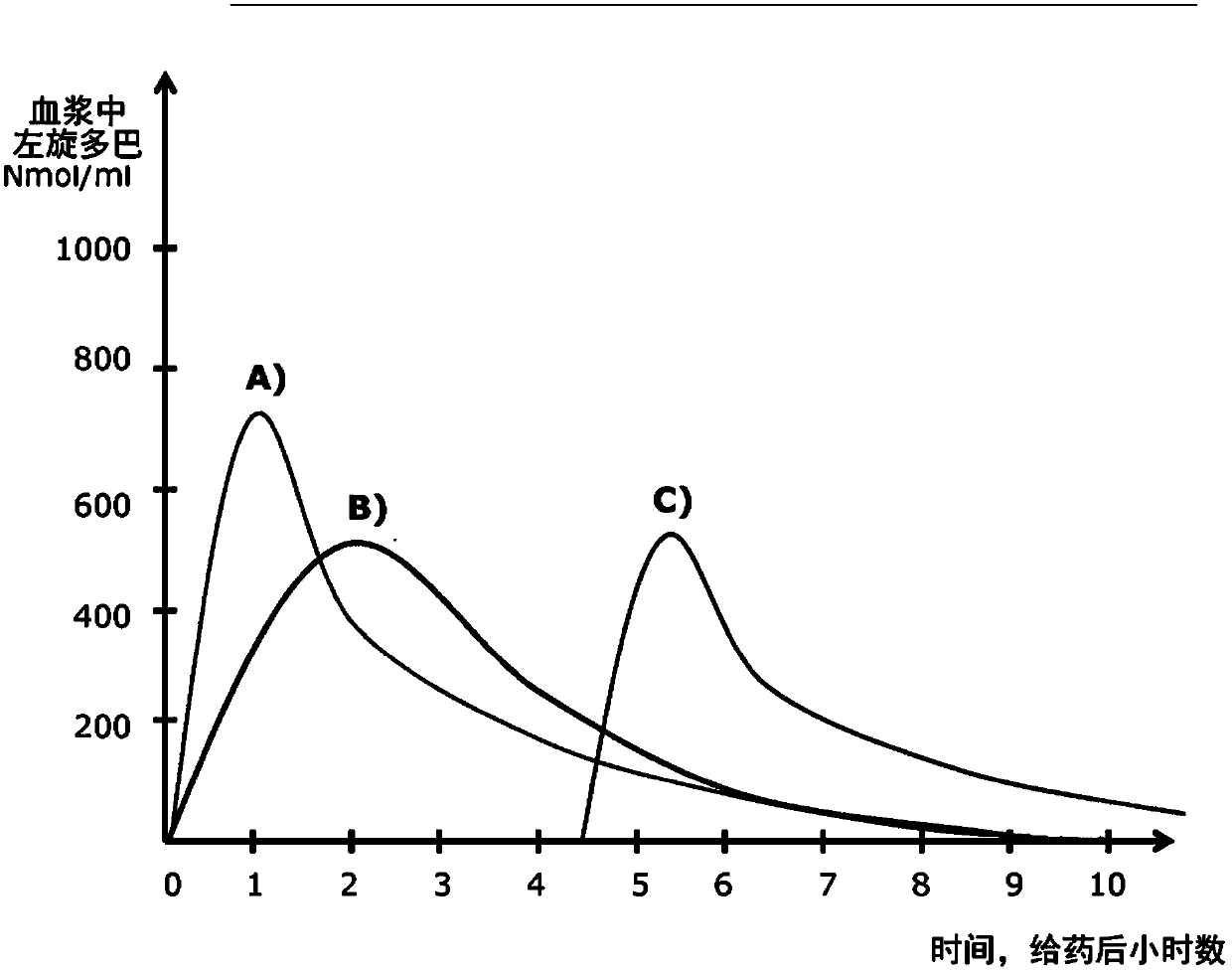
Pulsatile drug delivery system for treating morning akinesia
A drug and pulse release technology, applied in the directions of drug delivery, drug combination, capsule delivery, etc., can solve the problem of undetermined ER preparation scores, and achieve the effect of improving nighttime sleep patterns
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0280] Mix lactose with microcrystalline cellulose, sodium starch glycolate, and model compound (niacinamide) in a drum mixer for 5 minutes. Then magnesium stearate was added and mixed for 30 seconds. The mixture was compressed into tablets, each tablet having a weight of 6.6 mg, a size of 2 mm, and each tablet containing 0.28 mg of model compound. The tablet thickness is about 1.7 mm.
[0281] lactose
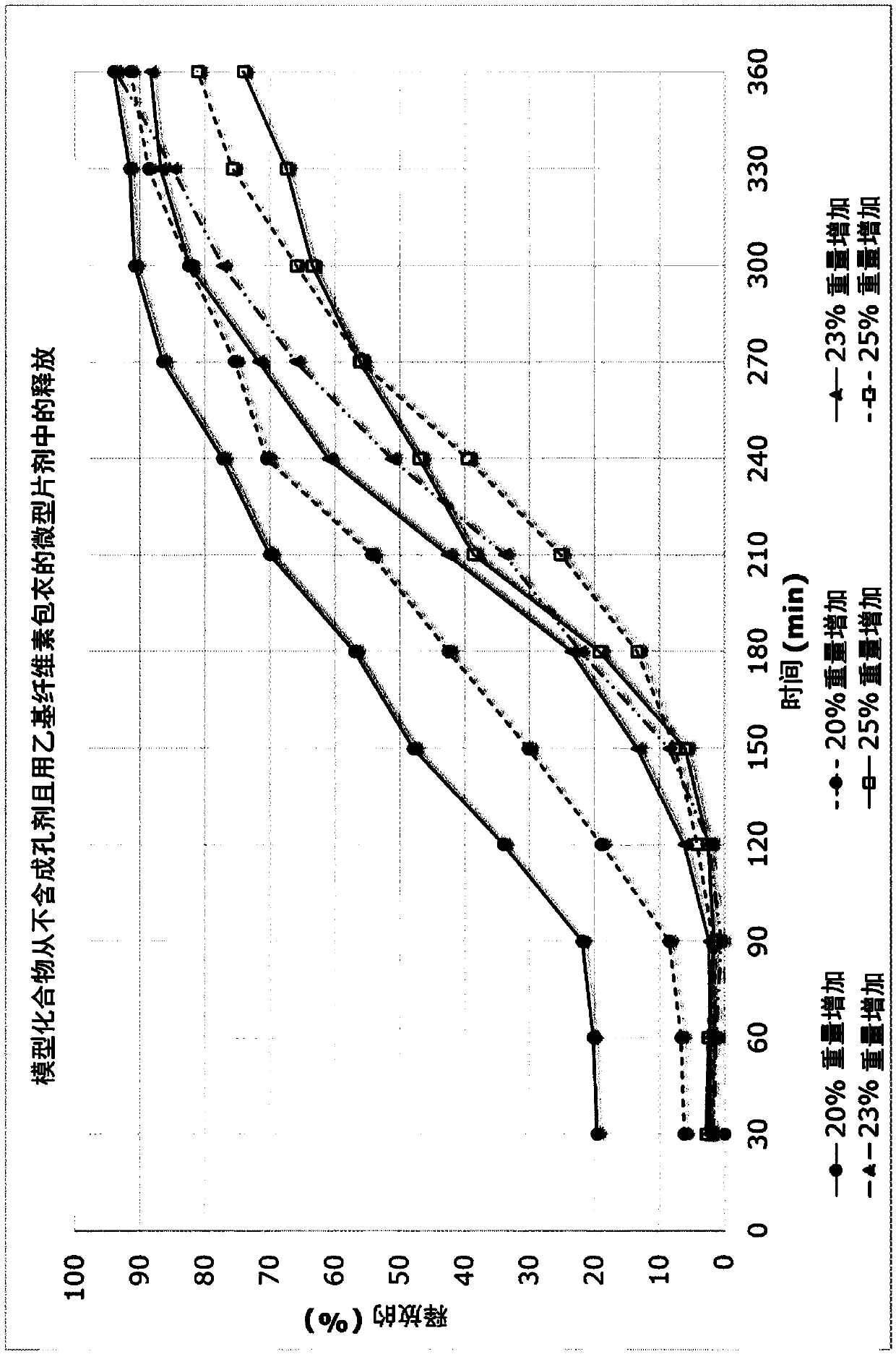
[0282] The model compound mini-tablets were film-coated with a semipermeable membrane based on ethylcellulose in a fluidized bed. The film composition is given in the table below. For 325g chips, 1000g film solution is produced to enable film coating to the desired tablet weight increase of up to 25.0%, including a 10% production loss excess. The spraying conditions are controlled so that the outlet air temperature is 28-30°C. To achieve the required weight gains of 20%, 23%, and 25%, 682.0 g, 784.9 g, and 853.1 g membrane solution were applied, respectively.
[0283] E...
Embodiment 2
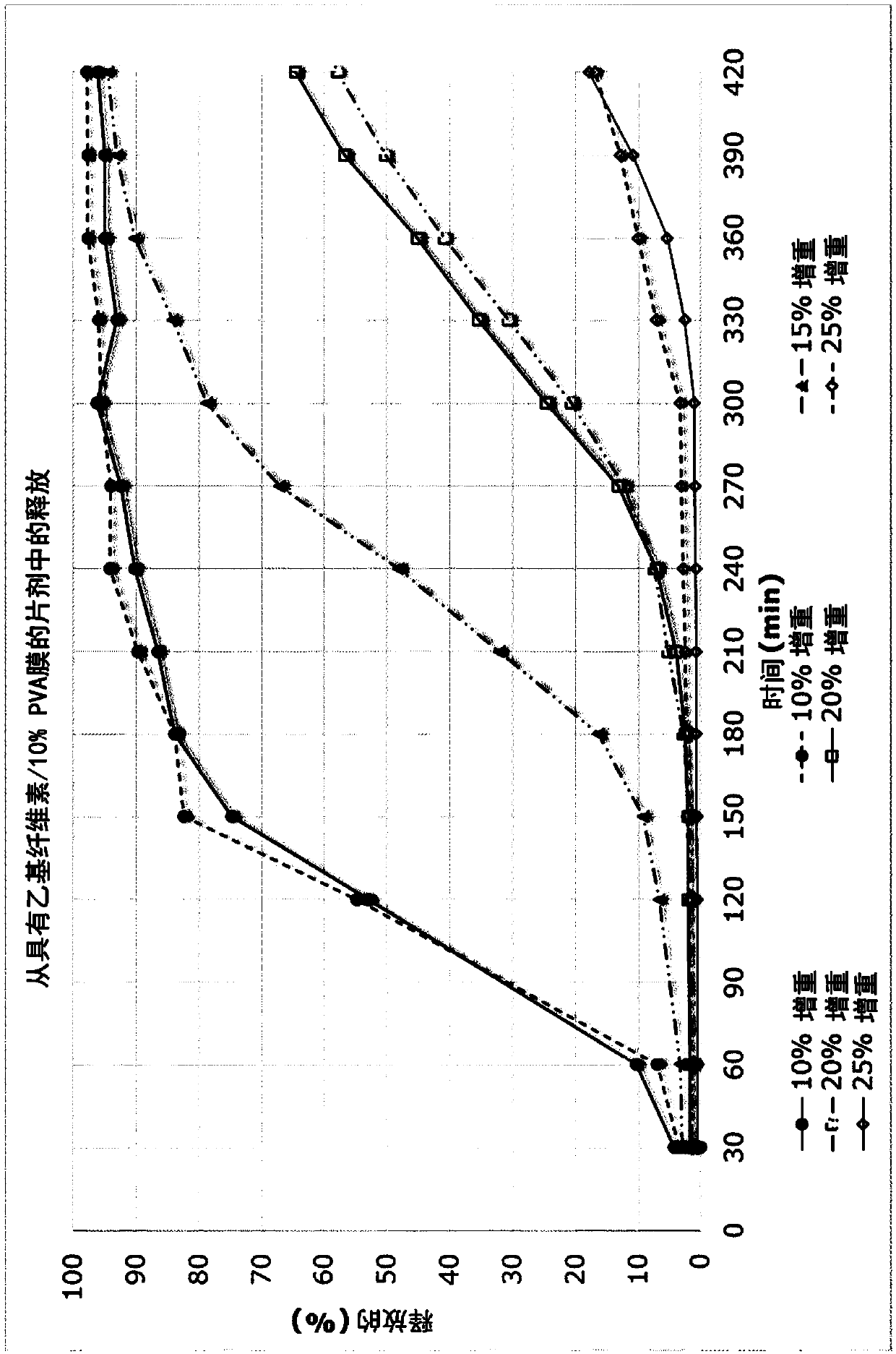
[0286] The microtablets of Example 1 were film-coated with an ethylcellulose-based semipermeable membrane in a fluidized bed. The film composition is given in the table below. For 325g chips, 1000g of membrane solution is produced to enable film coating to the required tablet weight increase of up to 25.0%, including a 10% production loss excess. The spraying conditions are controlled so that the outlet air temperature is 28-29°C. To achieve the required weight gains of 10%, 15%, 20%, and 25%, 341.3g, 511.9g, 682.5g, and 853.1g membrane solutions were applied, respectively.
[0287] Ethyl cellulose 7cps
[0288] The USP2 paddle device was used to test the dissolution of 44 microtablets. Each container contains 600 ml isotonic sodium chloride solution and is rotated at 75 rpm. The re-taken samples were quantified on a spectrophotometer at 260 nm. Result in Figure 3A Shown in.
Embodiment 3
[0290] As described in Example 2, the microtablets of Example 1 were film-coated with the following film composition:
[0291] Ethyl cellulose 7cps
[0292] Test the mini-tablets as described in Example 2 and the results are in Figure 3B Given in.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Sheet weight | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com