Escherichia coli expressing thermostable tyrosine phenolase and application thereof
A tyrosine phenol hydrolase and amino acid technology, applied in the field of Escherichia coli, can solve problems such as cytotoxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
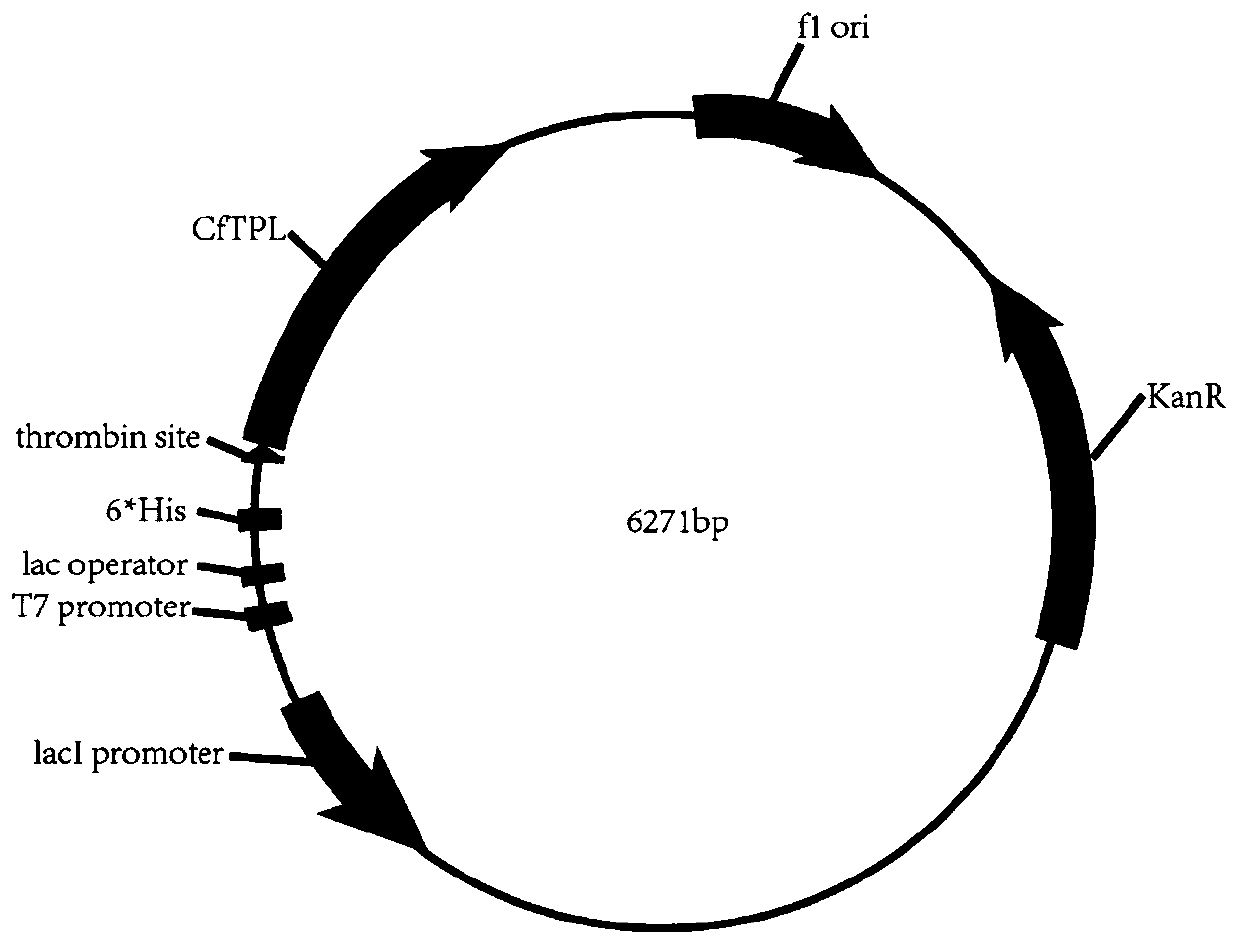
[0053] Example 1 Construction of recombinant plasmid pET-28(a)-TPL and 25 mutant recombinant plasmids.
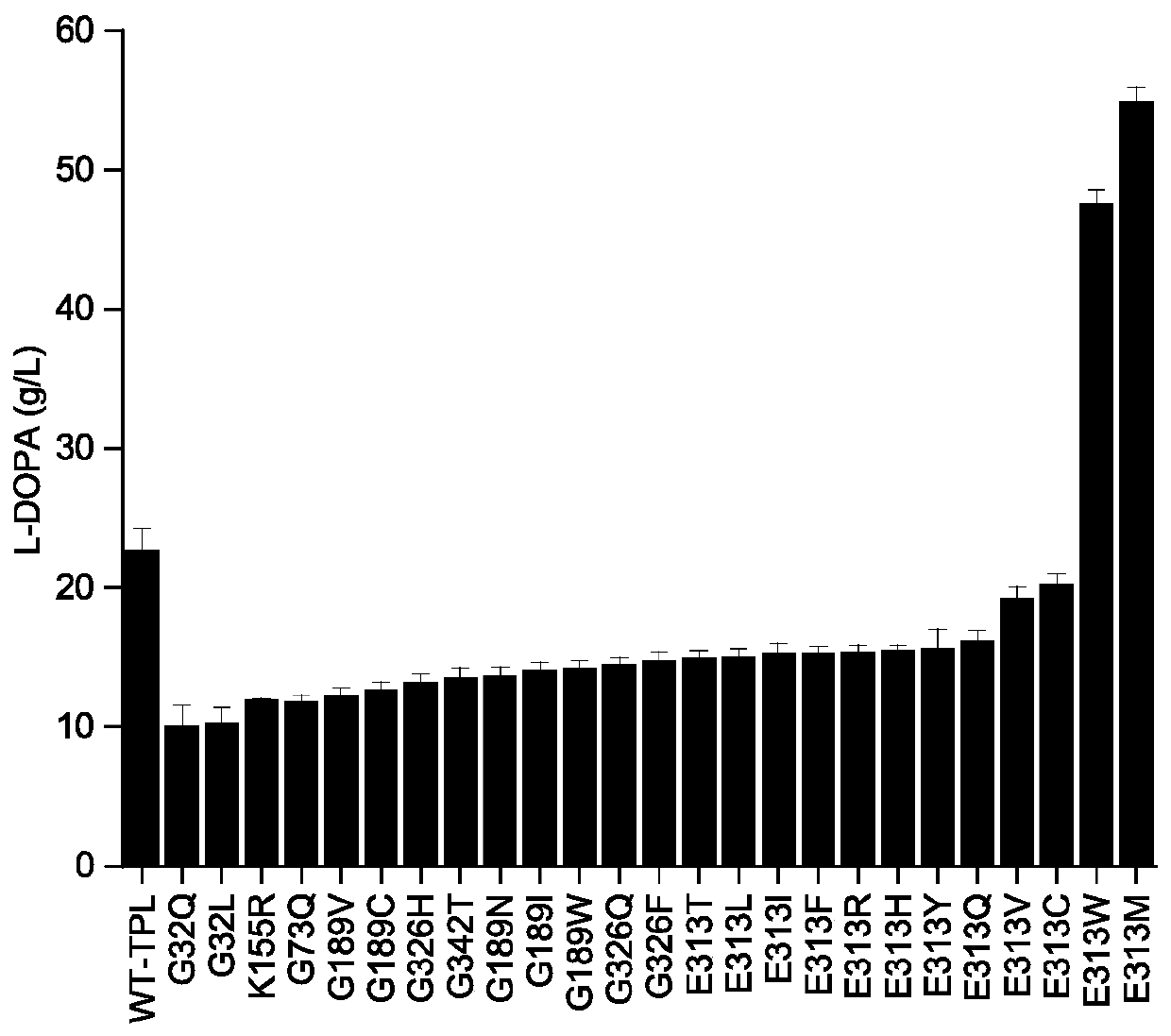
[0054] Based on the 3D model TPL-PLP (PDB: 2YHK, ), through the Calculate Mutation Energy (Stability) module of Discovery Studio (DS) around the active center of TPL The amino acids in the range were subjected to virtual mutation to determine that the key amino acids were Gly32, Gly73, Lys155, Gly326, Gly342, Gly189 and Glu313, and then the key amino acids were subjected to virtual saturation mutation through the Calculate Mutation Energy (Stability) module of Discovery Studio (DS), and the mutation The energy table (Table 1) predicted 25 strains expressing thermostable tyrosine phenolase.
[0055] Table 1 Mutation energy and predicted mutation effect
[0056]
[0057]
[0058] The tyrosine phenolase (TPL) gene (nucleotide sequence shown in SEQ ID NO.4) was synthesized by Nanjing Jinweizhi Co., Ltd., using the plasmid pET-28(a)+ as the expression vector, and the e...
Embodiment 2
[0066] Example 2 Recombinant Escherichia coli whole cell transformation method to produce L-DOPA
[0067] Inoculate Escherichia coli strains containing plasmid pET-28(a)-TPL and 25 mutated recombinant plasmids with correct sequencing results into LB plates (add thiokanamycin 50 mg / L), streak, and culture upside down at 37°C A large number of colonies grew in about 12 hours.
[0068] Inoculate a ring of single colonies to LB medium for seed culture, and culture at 220 rpm at 37°C for about 12 hours.
[0069] Inoculate the seed culture solution into the fermentation medium at an inoculum amount of 1%, culture at 37°C, 220rpm for 2h, add inducer 0.4mM IPTG and cool down to 20°C to continue fermentation for 10h, the growth of cells fermented by shake flasks of different strains is similar , OD 600 Both are around 25.
[0070] The prepared fermented broth was centrifuged at 6000 rpm for 10 min to collect wet cells. The prepared wet cells are added to the transformation liquid, ...
Embodiment 3
[0072] The expression situation of the tyrosine phenolase of embodiment 3 recombinant escherichia coli
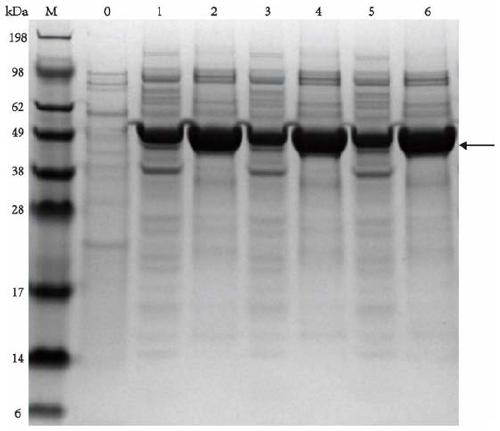
[0073] Centrifuge the fermentation broth of the strains containing plasmids pET-28(a)-TPL, pET-28(a)-TPL(E313W) and pET-28(a)-TPL(E313M) at 8000rpm for 3min to collect the cells, and use PB buffer (pH 8.5, 50mM KH 2 PO 4 -K2 HPO 4 ) Wash the cells 2-3 times, sonicate until the bacteria liquid is completely broken and become transparent, and centrifuge at 9000rpm for 3min to collect the supernatant. Protein purification was performed using a nickel column Ni-NTA Superflow Cabridge (5 mL) and an AKTA purifier. The purified protein solution was collected, desalted and purified using a desalting column Sephadex-G (2mL) and an AKTA purifier, and the protein concentration was detected by using the Enhanced BCA Protein AssayKit protein quantification kit (purchased from Biyuntian, item number: P0009), and analyzed by SDS- PAGE analysis of intracellular protein expression and p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com