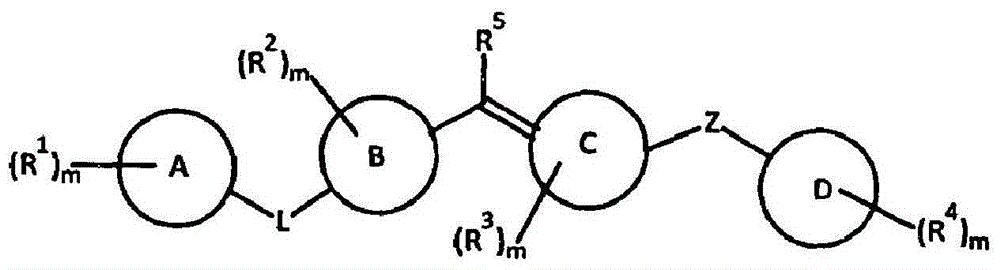
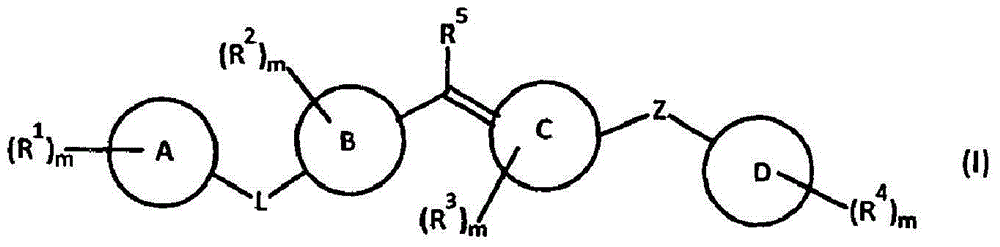
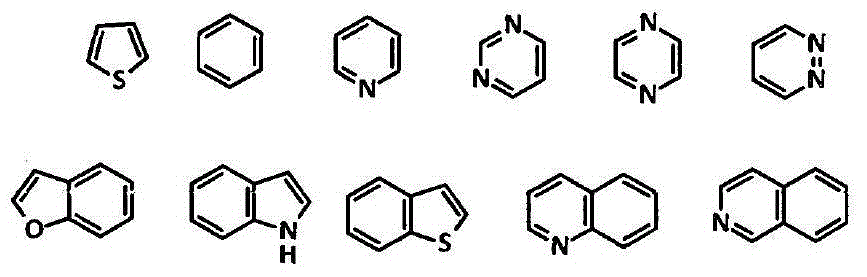
Amide compounds, compositions and applications thereof
A compound and solvate technology, which is applied in the field of amide compounds, compositions and their applications, can solve the problems of cognitive impairment, motor control, and limitation of wide clinical application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 11
[0706] Example 1.1N-(5-chlorothiazol-2-yl)-4-[[3-[[5-(trifluoromethyl)-2-pyridyl]oxy]phenyl]methylene]cyclohexyl Alkane carboxamide
[0707]
[0708] 4-[[3-[[5-(trifluoromethyl)-2-pyridyl]oxy]phenyl]-methylene]cyclohexanecarboxylic acid (intermediate 1A) (1.0g , 2.7 mmol) in DCM (20 mL) was added DMF (0.01 mL) followed by oxalyl chloride (0.5 mL, 5.3 mmol). After stirring at room temperature for 2 h, the volatiles were evaporated. The resulting residue was dissolved in DCM (5 mL) at 0 °C and a solution of 2-amino-5-chlorothiazole hydrochloride (Intermediate 2A) (680 mg, 4.0 mmol) in DCM (15 mL) was added. Triethylamine (1.1 mL, 8.0 mmol) was added thereto and stirred for 2 h. Water (30 mL) was added to quench the reaction. The organic layer was diluted with DCM (50 mL), separated, washed with brine (30 mL), dried over anhydrous sodium sulfate, filtered, concentrated and purified by column chromatography to give 400 mg (34%) of the title compound as a solid.
[0709] 1...
Embodiment 12
[0713] Example 1.2: N-(3-pyridyl)-4-[[3-[[5-(trifluoromethyl)-2-pyridyl]oxy]phenyl]-methylene]cyclohexane Amide hydrochloride
[0714]
[0715] 4-[[3-[[5-(trifluoromethyl)-2-pyridyl]oxy]phenyl]-methylene]cyclohexanecarboxylic acid (300mg, 0.8mmol) in DCM at 0°C (50 mL) was added DMF (0.01 mL) followed by oxalyl chloride (0.2 mL, 1.6 mmol). Stir at room temperature for 2h and evaporate the volatiles. The resulting residue was dissolved in DCM (2 mL) at 0 °C and a stirred solution of 3-aminopyridine (Intermediate 2F) (75 mg, 0.8 mmol) in DCM (3 mL) was added. Triethylamine (0.1 mL, 0.9 mmol) was added thereto and stirred for 2 h. Water (10 mL) was added to quench the reaction. The organic layer was diluted with DCM (10 mL), separated, washed with brine (30 mL), dried over anhydrous sodium sulfate, filtered, concentrated and purified by column chromatography. The resulting product was stirred with 4N HCl in dioxane (5 ml) for 1 h. Excess solvent was evaporated under redu...
Embodiment 21
[0717] Example 2.1: N-[4-[[3-[[5-(trifluoromethyl)-2-pyridyl]oxy]phenyl]methylene]cyclohexyl]pyridine-3-carboxamide
[0718]
[0719] 4-[[3-[[5-(trifluoromethyl)-2-pyridyl]oxy]phenyl]methylene]cyclohexylamine (Intermediate 3A) (200mg, 0.57mmol) at 0°C and a mixture of nicotinoyl chloride (Intermediate 4A) (150 mg, 0.86 mmol) in DCM (10 mL) was slowly added triethylamine (0.5 mL, 3.5 mmol). After stirring at room temperature for 2 h, the volatiles were evaporated under reduced pressure. The resulting residue was taken up in ethyl acetate (25 mL) and washed with anhydrous saturated sodium bicarbonate solution (20 mL), water (20 mL), brine (20 mL), dried over anhydrous sodium sulfate, filtered, concentrated and purified by preparative HPLC , thus giving 75 mg (30%) of N-[4-[[3-[[5-(trifluoromethyl)-2-pyridyl]oxy]phenyl]methylene]cyclohexyl]-pyridine -3-Carboxamide.
[0720] 1 H NMR (400MHz, CDCl 3 ): δ1.37(qd, J=11.0, 5.6Hz, 1H), 1.48(qd, J=11.3, 5.1Hz, 1H), 2.15-2.59(m, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com