Endomorphin analogue and application thereof
A technology of endomorphin and analogues, applied in the field of endomorphin analogues and its application, can solve problems such as withdrawal symptoms, respiratory depression, addiction, etc., achieve improved imbalance, good anti-enzymolysis effect, and improve side effects Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
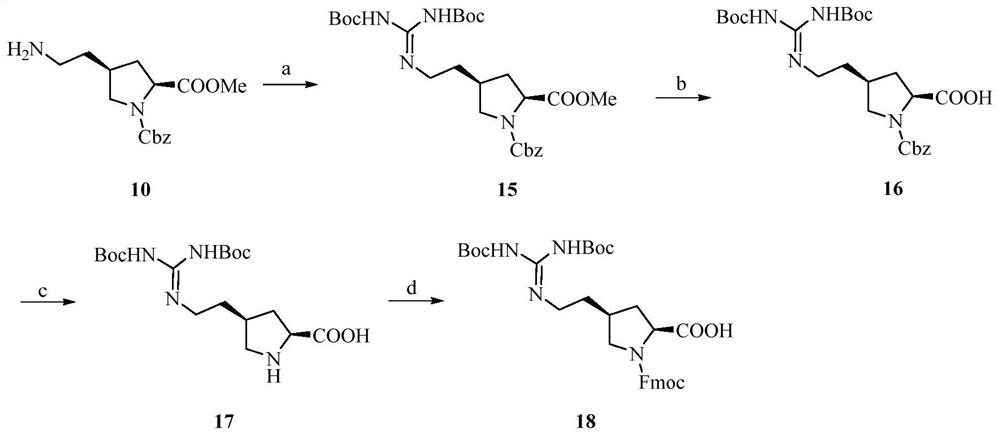
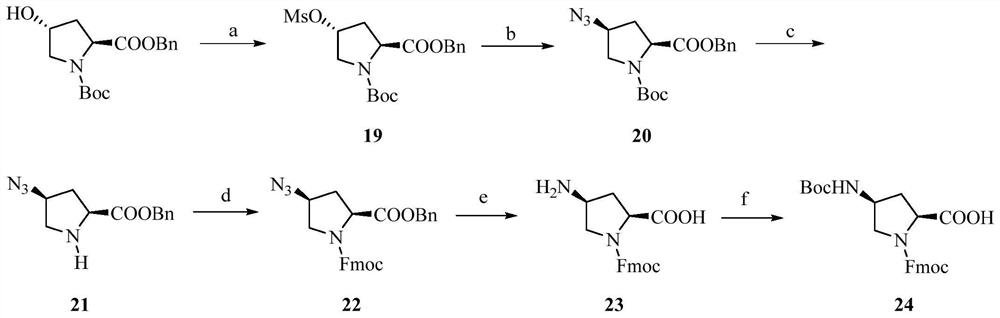
Image
Examples
Embodiment 1
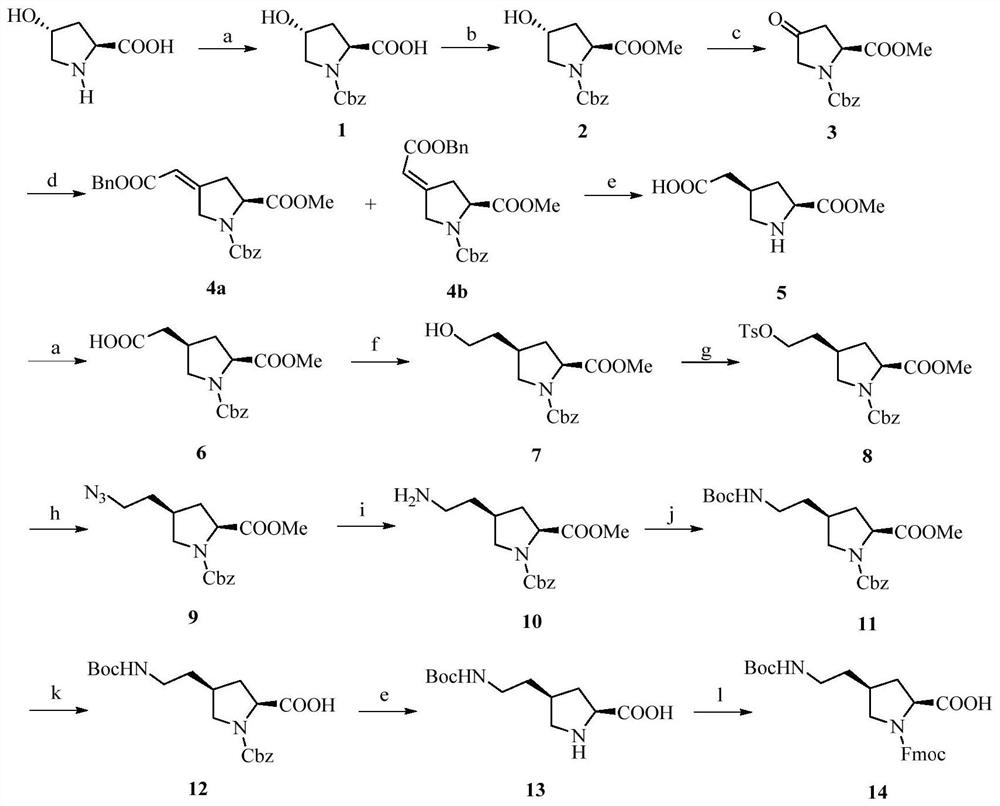
[0024] The synthesis of embodiment 1 anti-N-benzyloxycarbonyl-4-hydroxyl-proline (1)
[0025] 4-Hydroxyproline (10g, 76.4mmol) was dissolved in saturated NaHCO 3 (376mL) and THF (376mL), under ice-cooling conditions, dropwise add benzyl chloroformate (15.4g, 91.6mmol) THF (20mL) solution, remove the ice-bath, and stir overnight. After the reaction is complete, adjust the pH to 3 with 3mol / L hydrochloric acid, extract with EA, collect the organic layer, anhydrous Na 2 SO 4 dry. Rotary evaporation gave 25 g of colorless oily liquid. It was directly used for the next reaction without purification.
Embodiment 2
[0026] Synthesis of embodiment 2 anti-N-benzyloxycarbonyl-4-hydroxyl-proline methyl ester (2)
[0027] Add SOCl to 300 mL of methanol solution under ice bath condition 2 (12.5mL, 164mmol), a small amount of methanol (15mL) dissolved compound 1 (25g, 94mmol), added to the above system, stirred for 10min, removed the ice bath, refluxed overnight. After the reaction stopped, spin dry. Silica gel column chromatography (PE:EA=3:1-1:1) gave 22.5 g of a colorless oily substance, with a yield of 85.7%.
Embodiment 3
[0028] Example 3 Synthesis of N-benzyloxycarbonyl-4-oxo-proline methyl ester (3)
[0029] Compound 2 (10g, 35.8mmol) was dissolved in dichloromethane (200mL), trichloroisocyanic acid (6.3g, 27.3mmol) was added in batches, and stirred for 15min; at 0°C, TEMPO (0.58g, 3.7mmol) was added in batches ), stirred for 2h. After the reaction is complete, use saturated NaHCO 3 , 10% citric acid, water, saturated NaCl washing, anhydrous Na 2 SO 4 dry. After rotary evaporation, the crude product was subjected to silica gel column chromatography (PE:EA=3:1-1:1) to obtain 7.45 g of light yellow oil with a yield of 75.1%. 1 H NMR (400MHz, CDCl 3 )δ7.35–7.32(m,5H),5.24–5.10(m,2H),4.87–4.81(m,1H),3.98–3.90(m,2H),3.76–3.62(m,3H),2.95– 2.89(m,1H),2.63–2.57(m,1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com