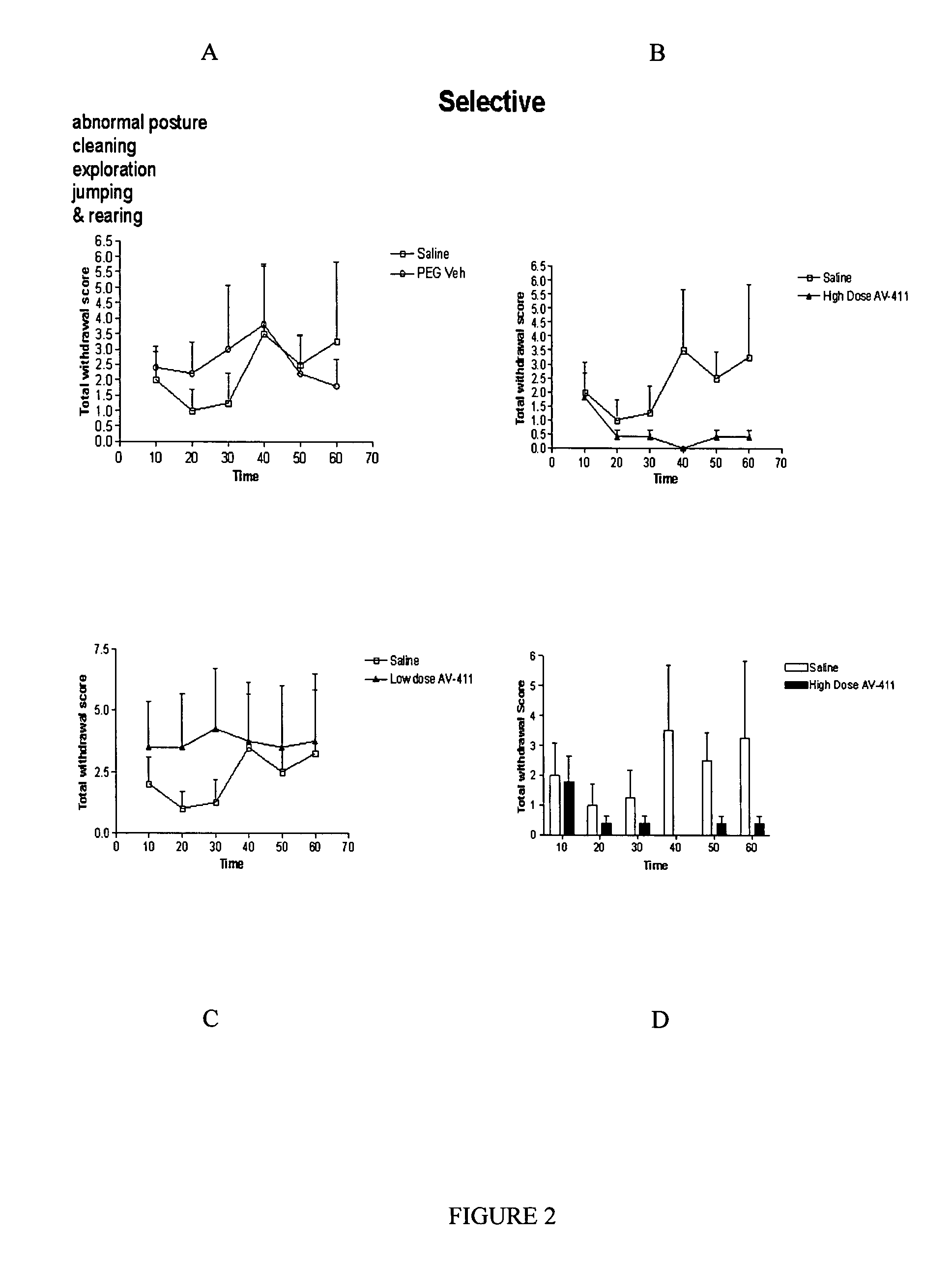
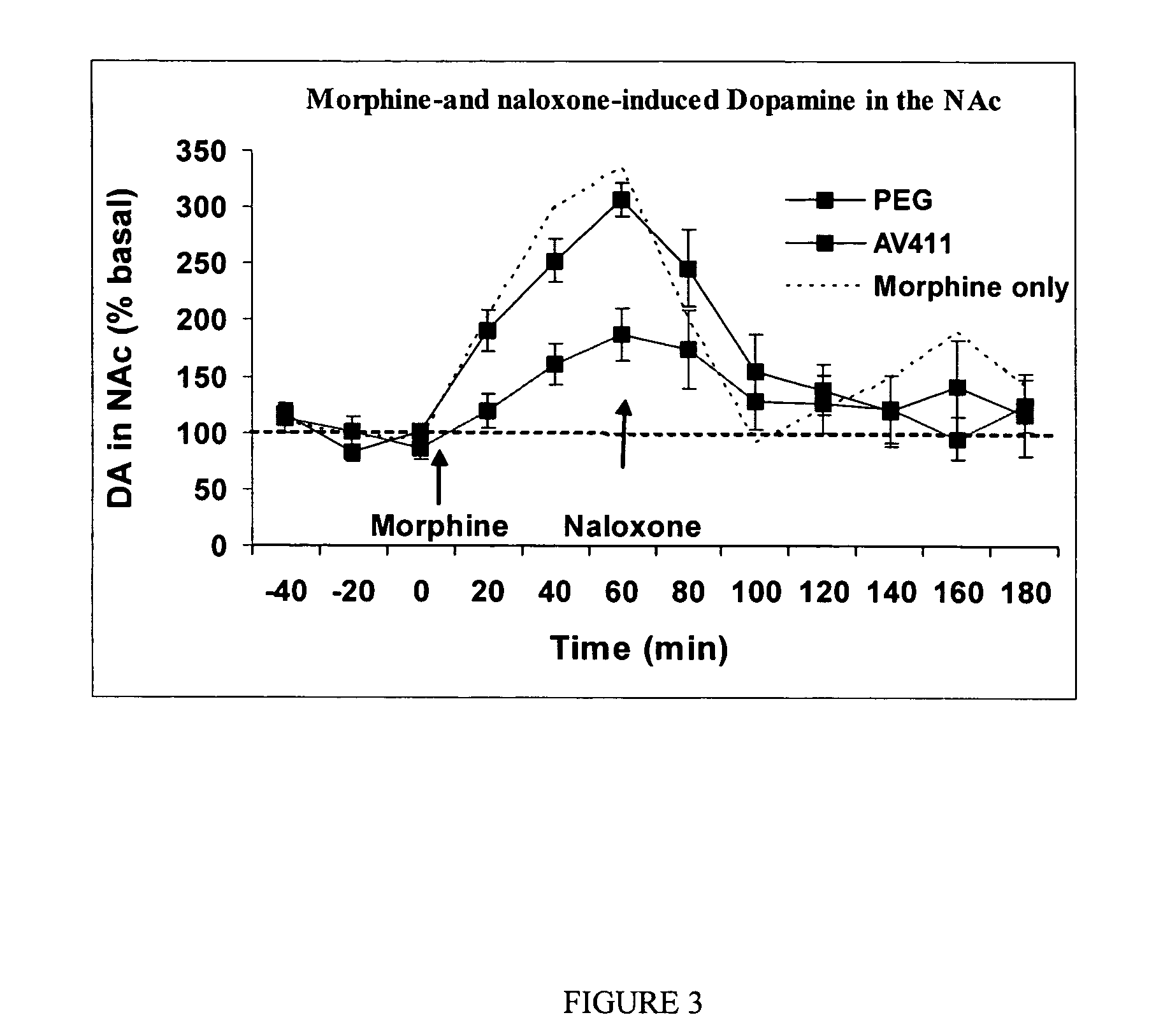
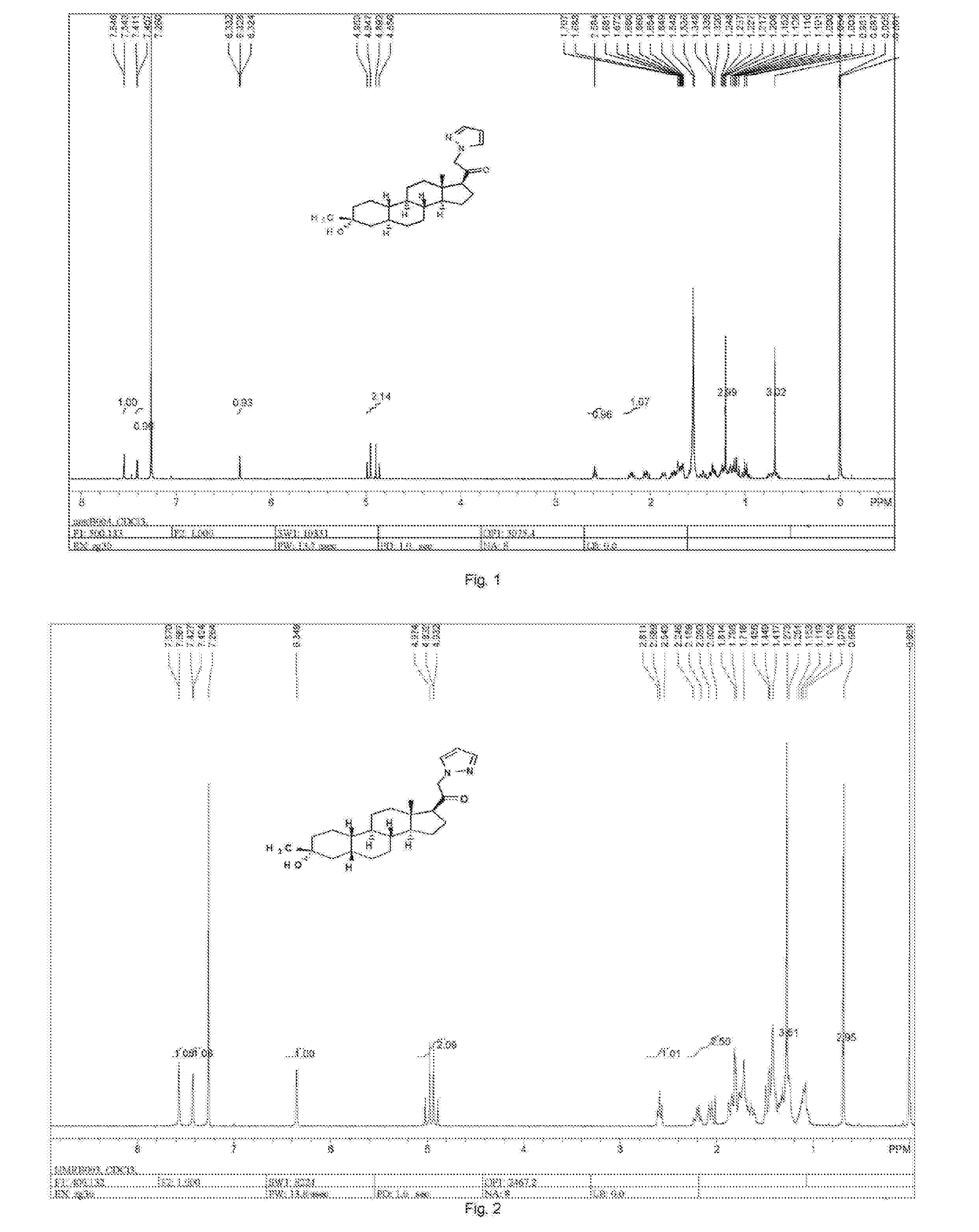
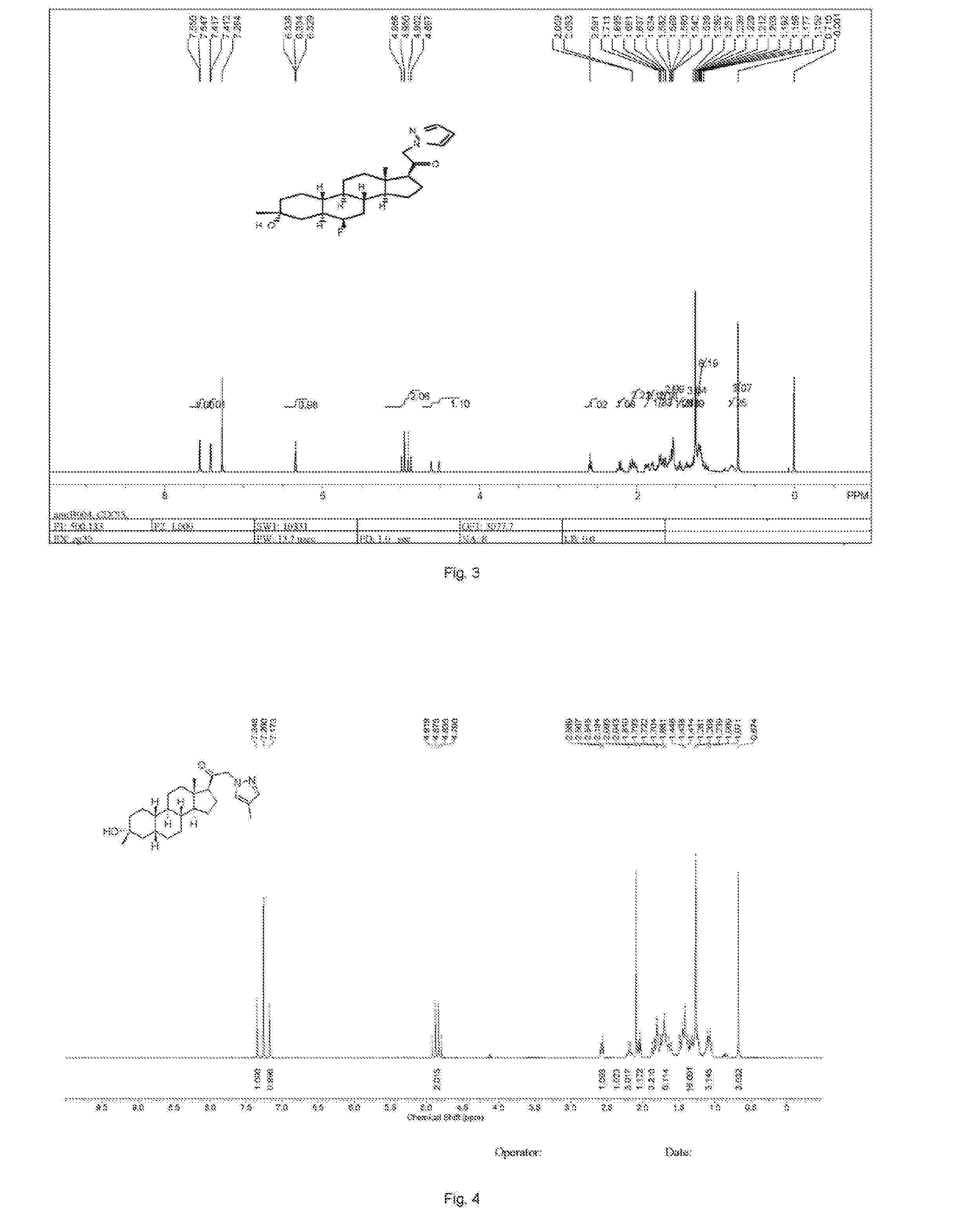
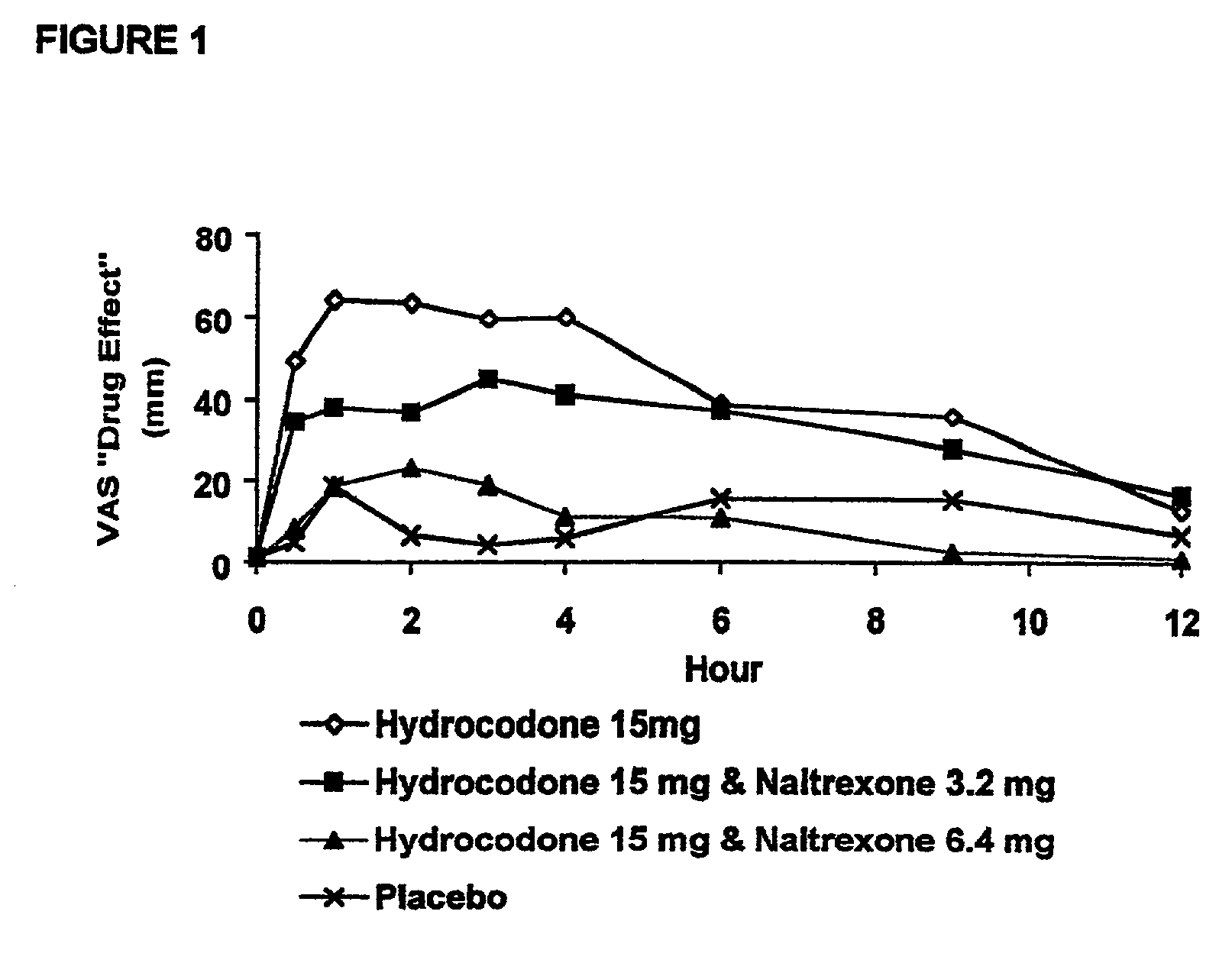
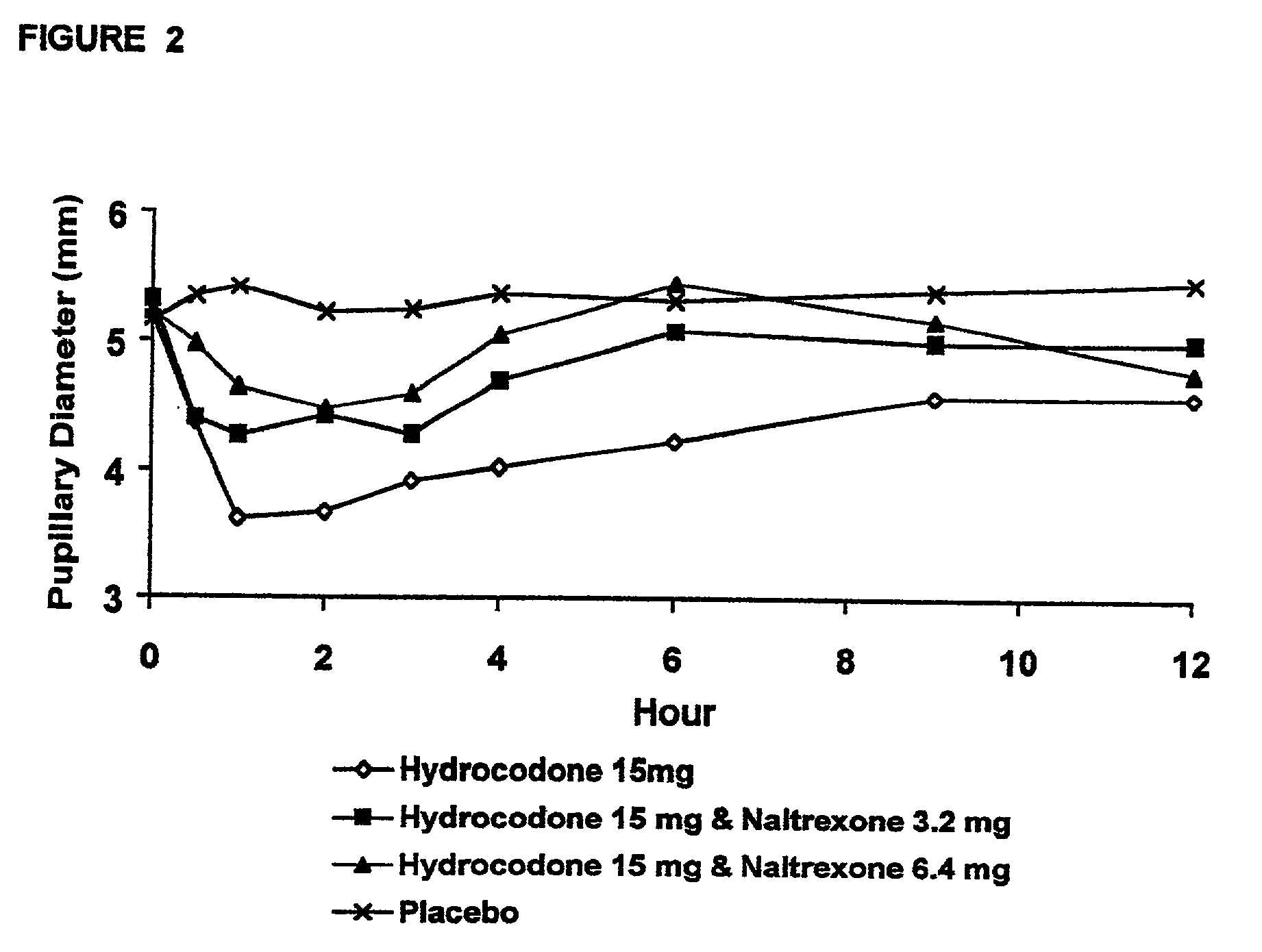
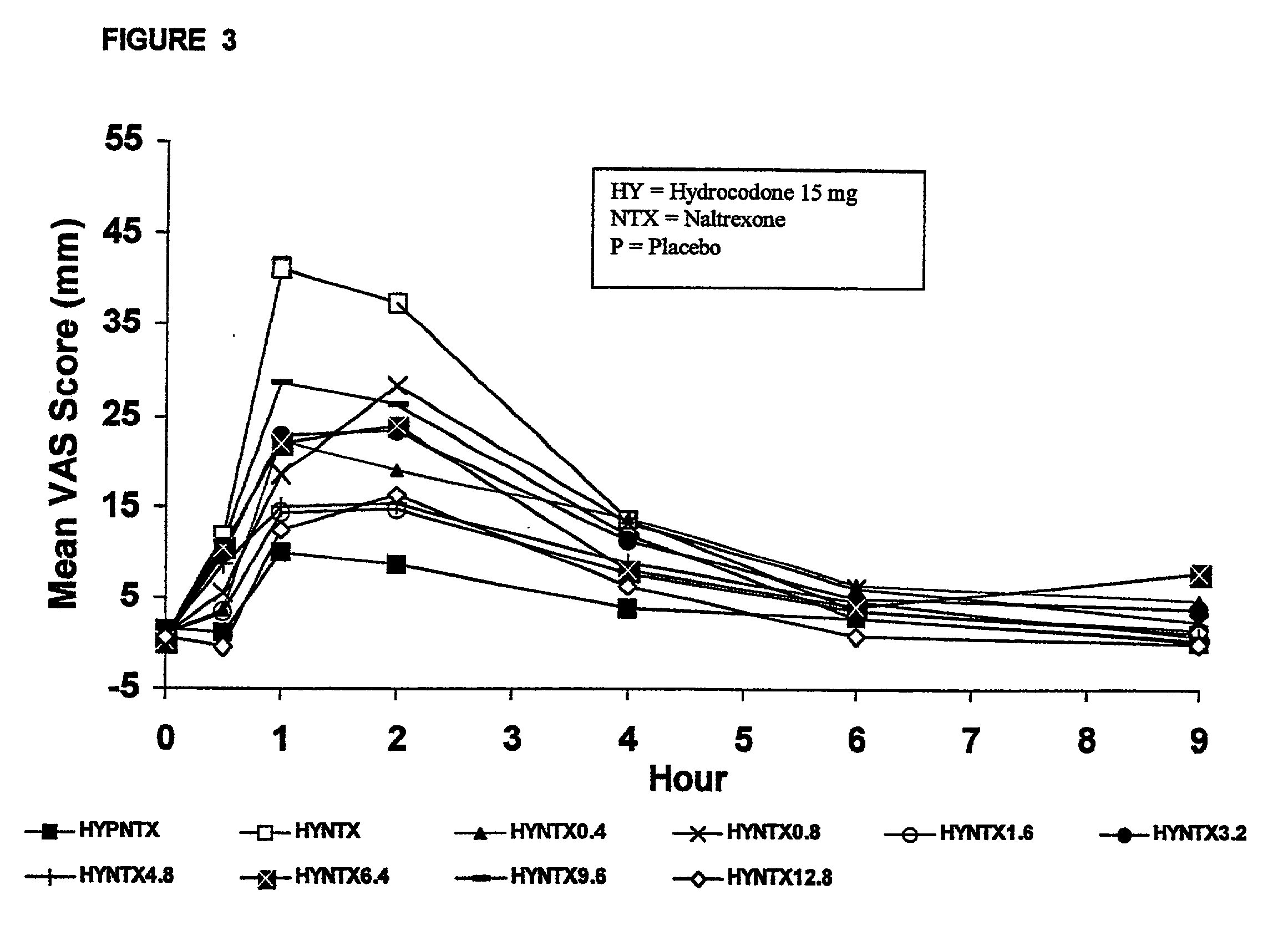
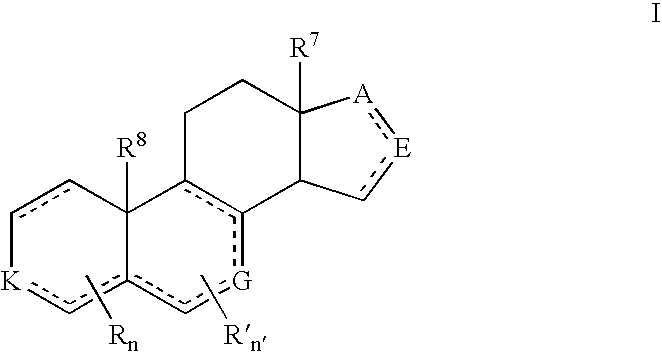
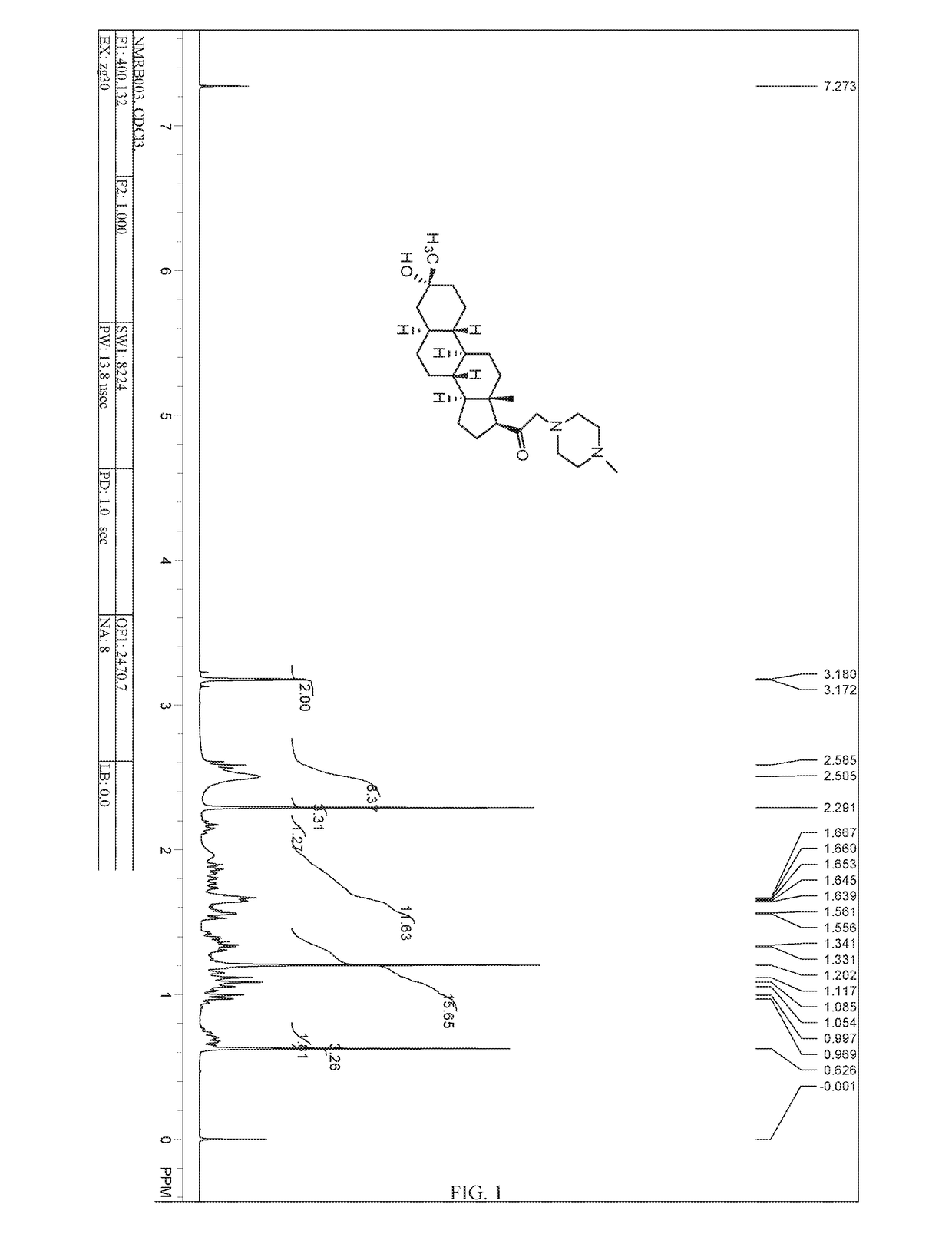
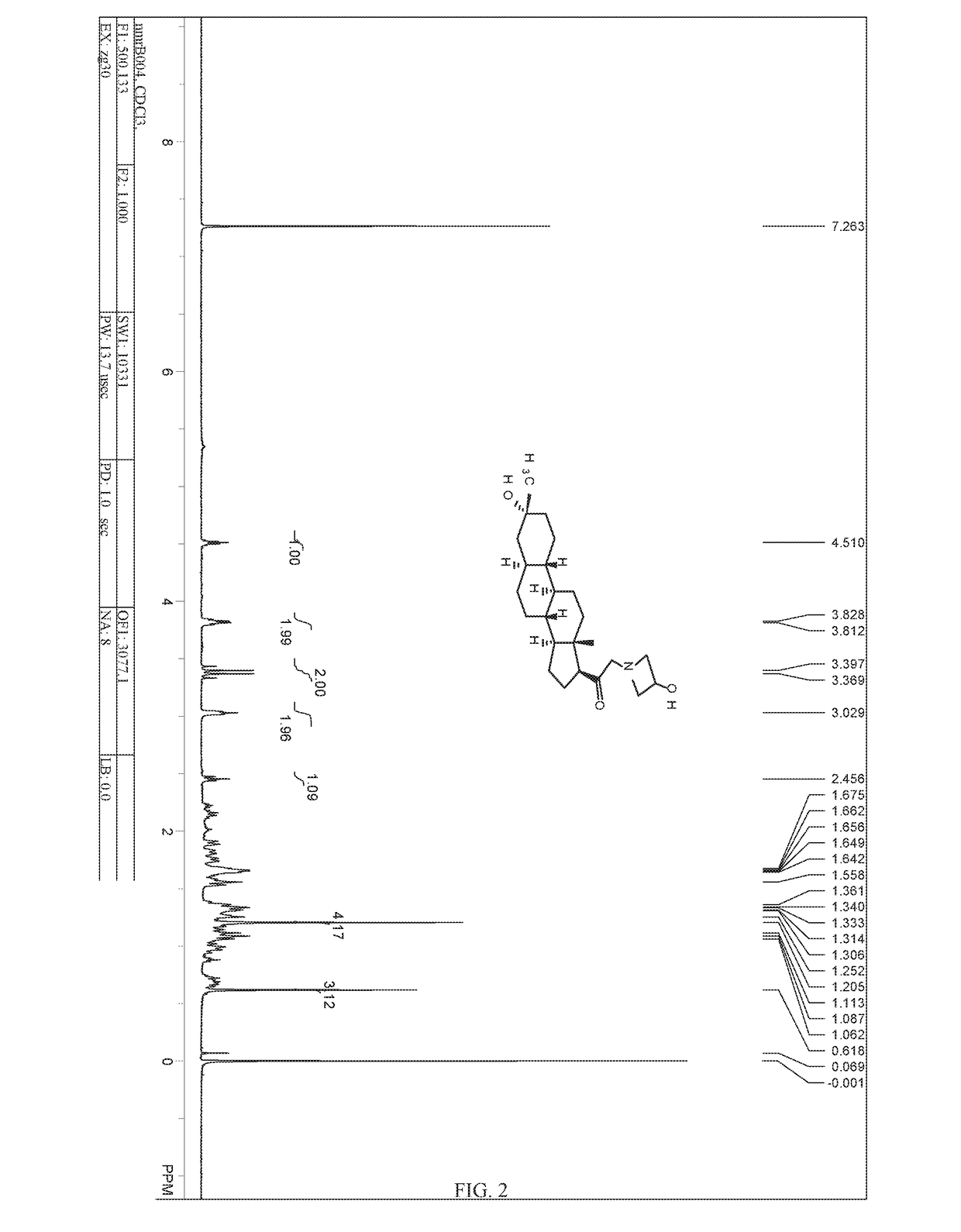
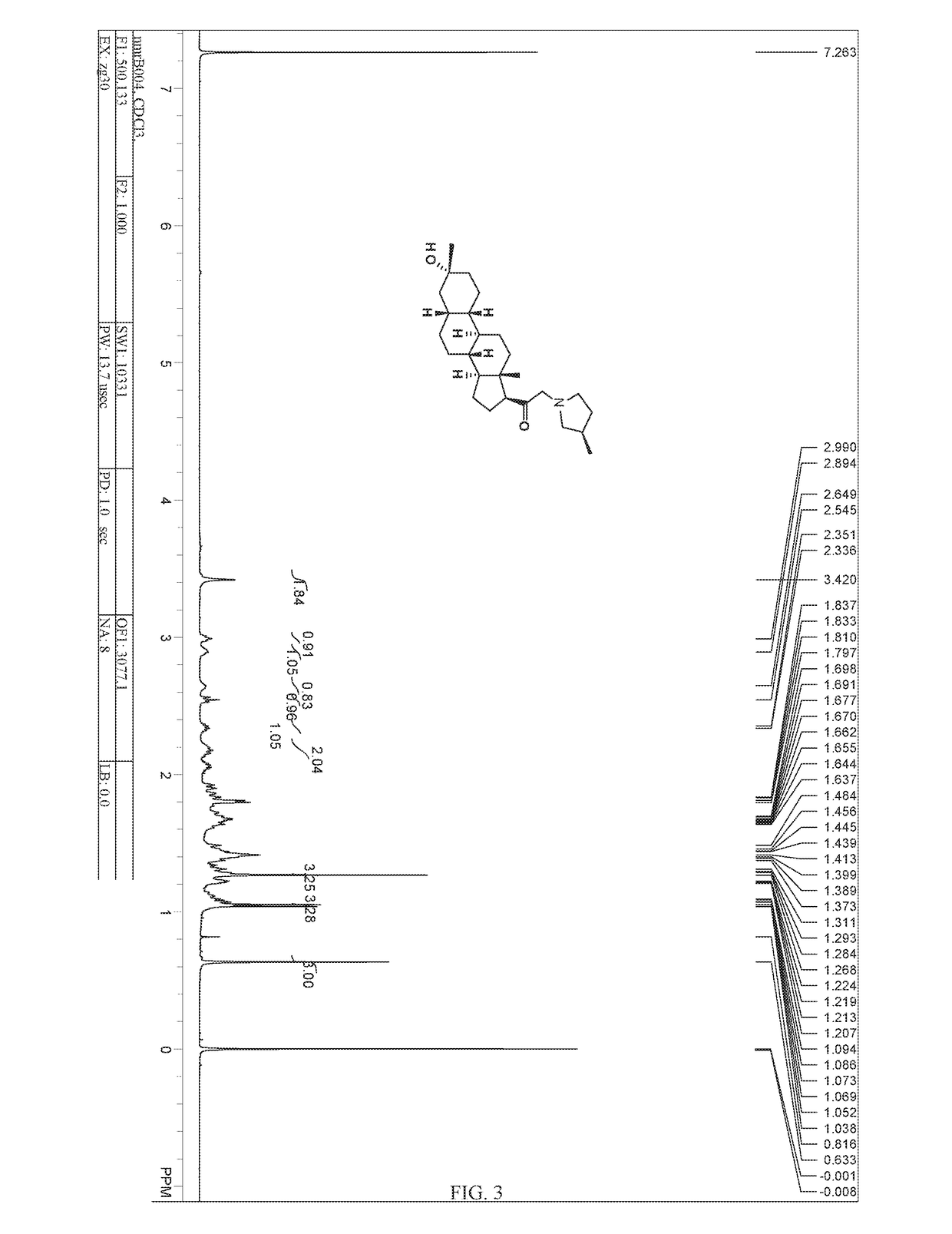
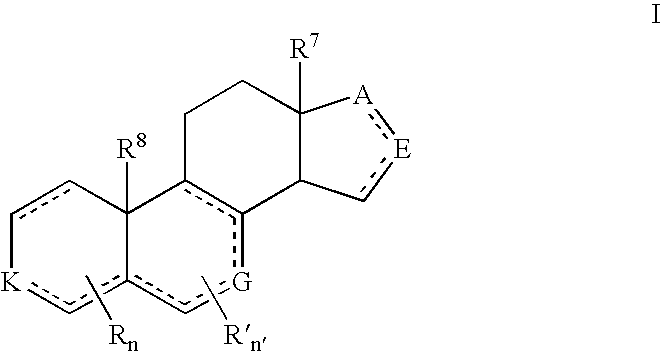
Patents
Literature
45 results about "Drug withdrawal syndrome" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Definition Withdrawal syndrome occurs in drug and alcohol addicted individuals who discontinue or reduce the use of their drug of choice. This process of eliminating drugs and alcohol from the body is known as detoxification. Drugs and alcohol affect mood by altering brain chemistry, specifically the production of neurotransmitters.
Opioid agonist/antagonist combinations
InactiveUS6277384B1Increase elasticityTrend downBiocideNervous disorderOpioid AgonistOpioid antagonist
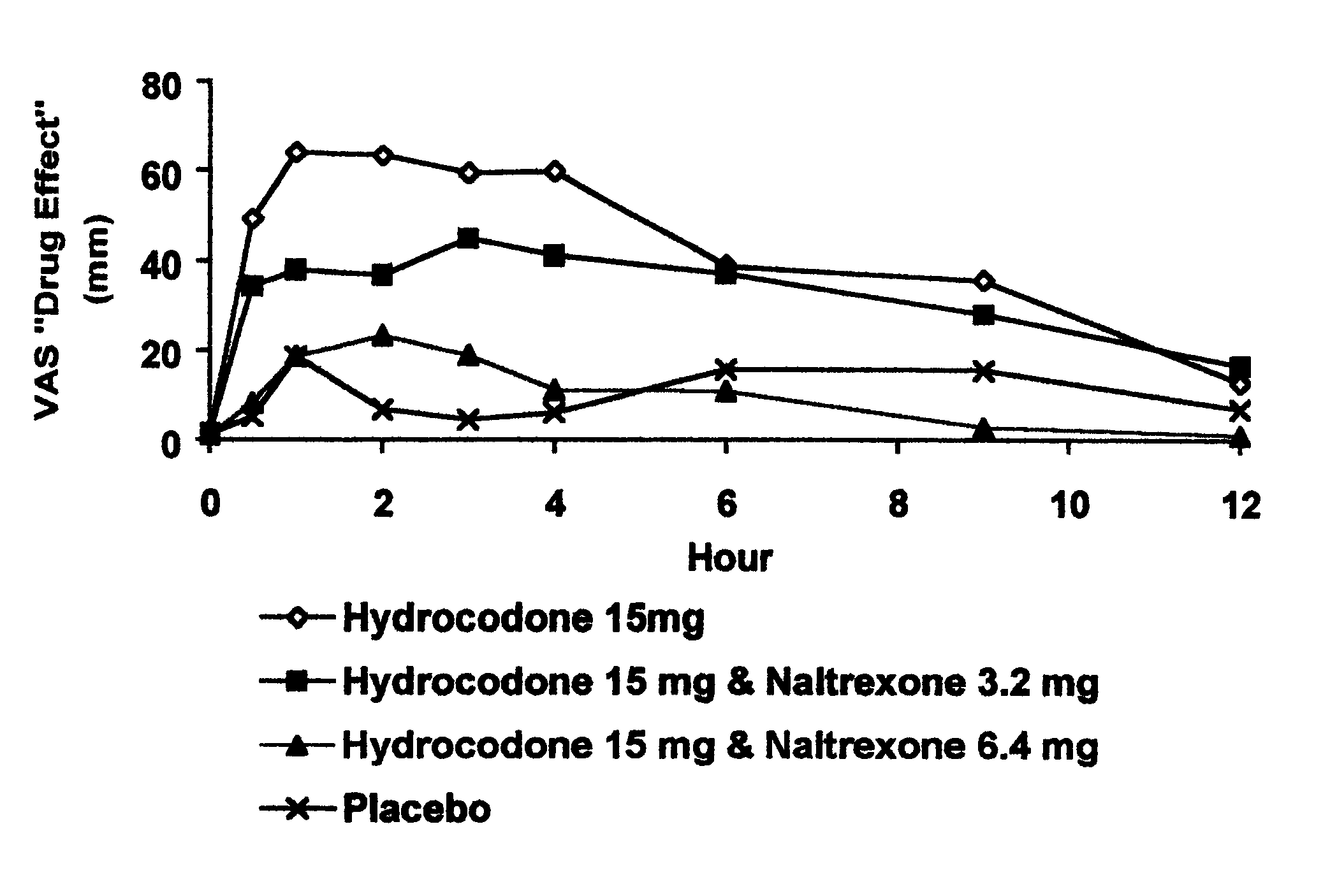
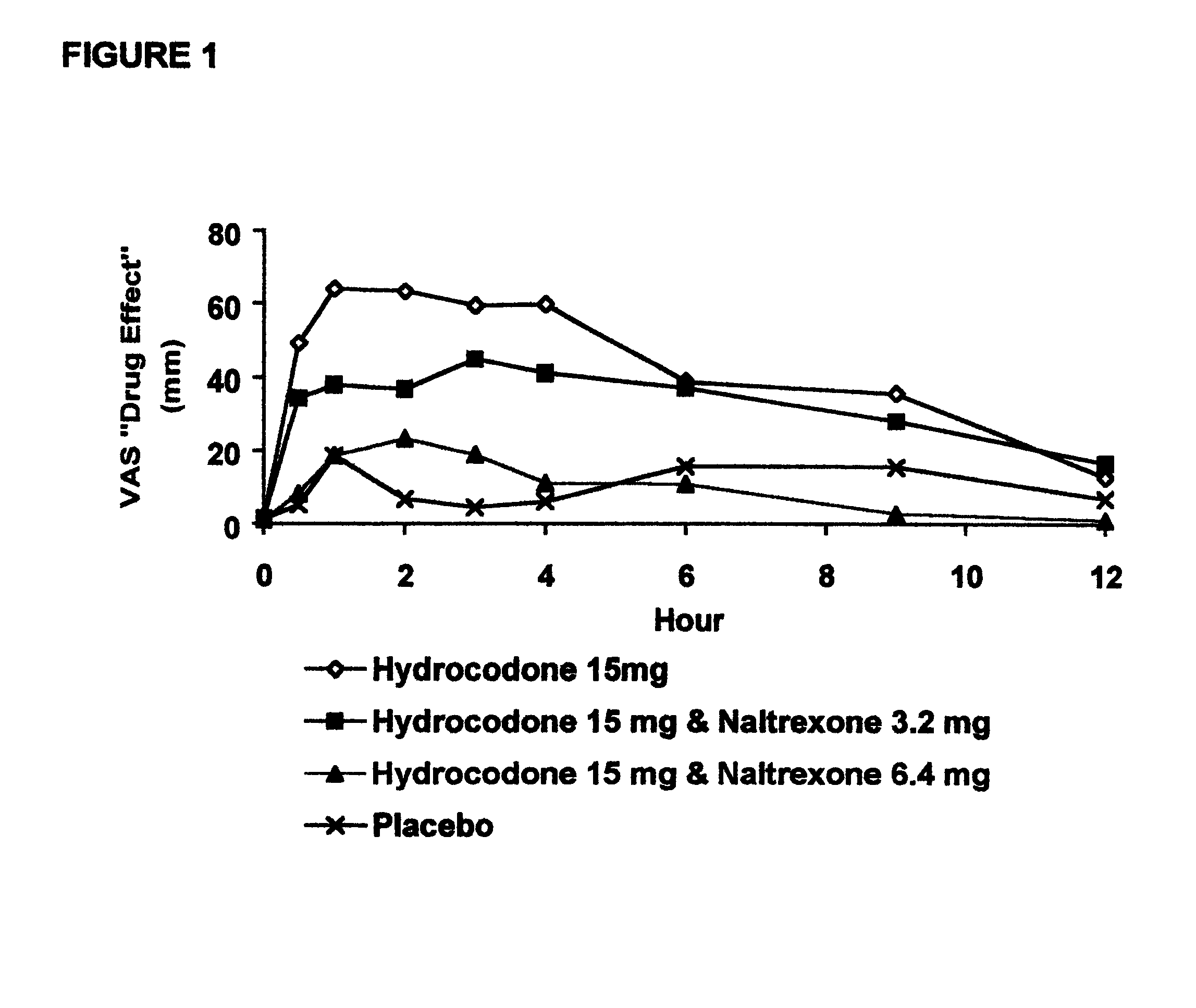
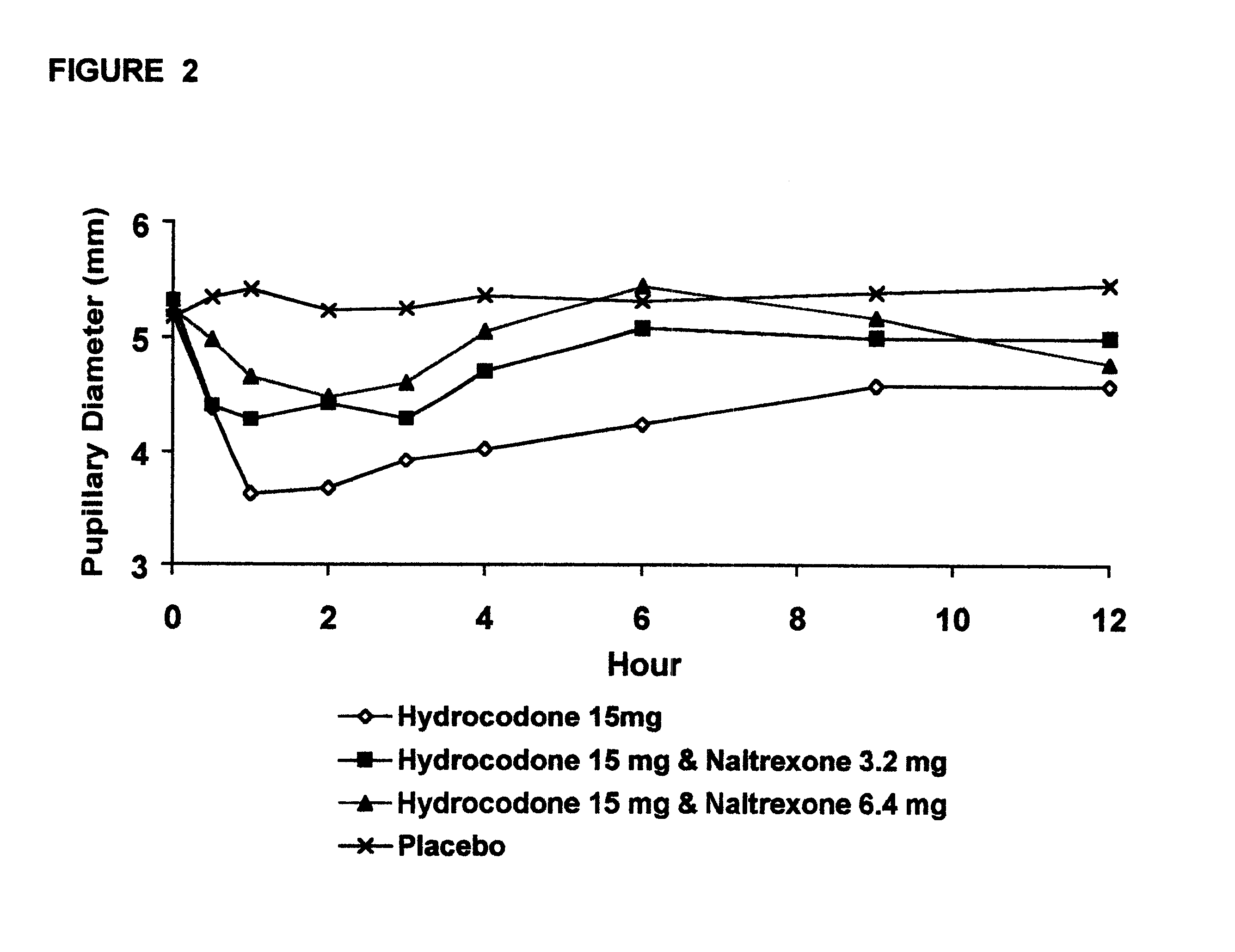
The invention is directed in part to oral dosage forms comprising a combination of an orally analgesically effective amount of an opioid agonist and an orally active opioid antagonist, the opioid antagonist being included in a ratio to the opioid agonist to provide a combination product which is analgesically effective when the combination is administered orally, but which is aversive in a physically dependent subject. Preferably, the amount of opioid antagonist included in the combination product provides at least a mildly negative, "aversive" experience in physically dependent addicts (e.g., precipitated abstinence syndrome).
Owner:PURDUE PHARMA LP
Method for treating drug and behavioral addictions
ActiveUS20070072899A1Diminishing and eliminating symptomReduce weight lossBiocideNervous disorderWithdrawal syndromeAddictive drugs
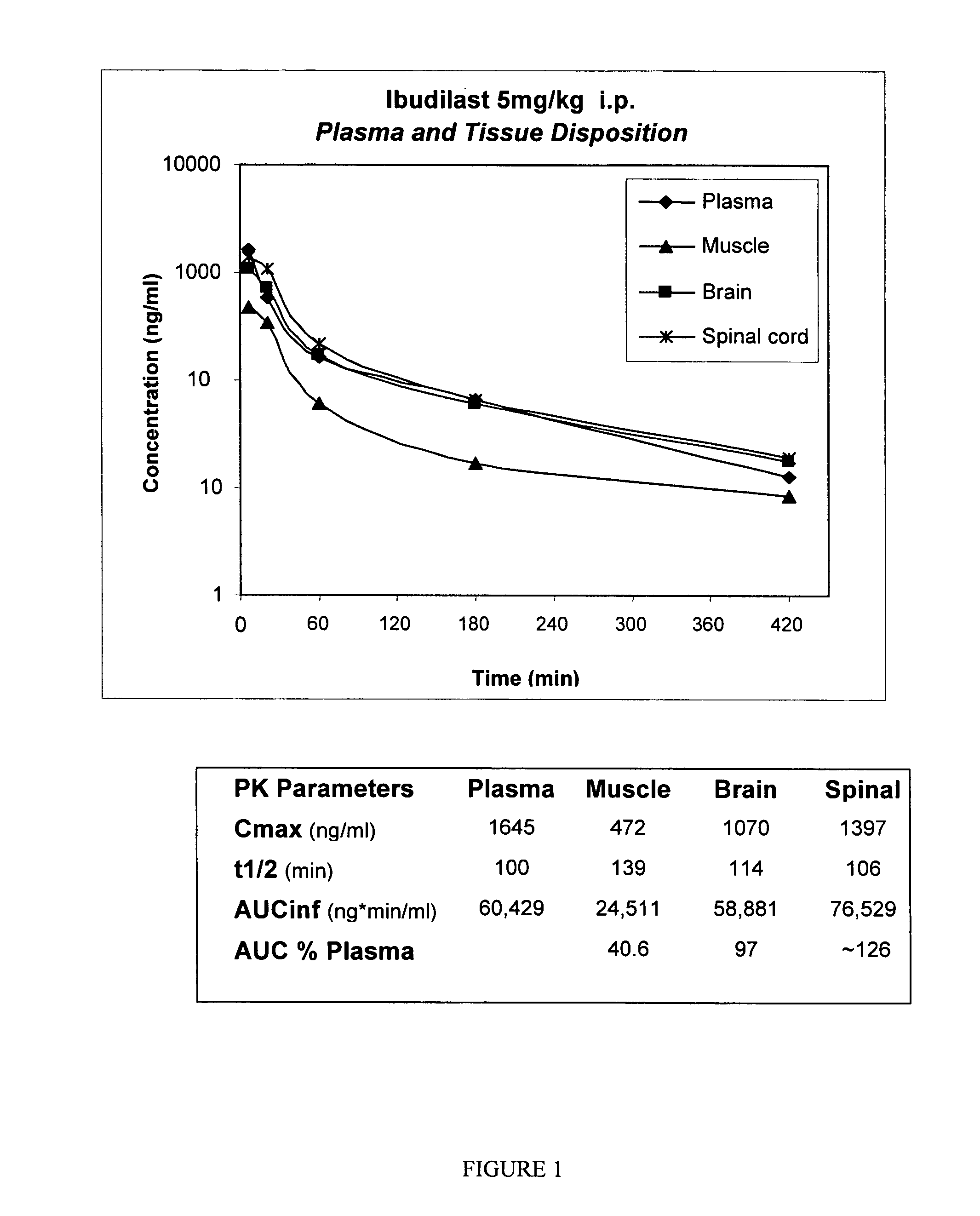
The present invention is directed to the use of ibudilast for treating addictions, including drug and behavioral addictions. In particular, ibudilast is used to diminish the dopamine-mediated reward associated with addictions and to treat withdrawal syndromes after discontinuance of addictive drug use or behavior.
Owner:MEDICINOVA INC +1
19-nor C3, 3-disubstituted C21-N-pyrazolyl steroids and methods of use thereof
ActiveUS9512165B2Eliminate potential for oxidationImprove bioavailabilityOrganic active ingredientsSenses disorderSubstance abuserWithdrawal syndrome
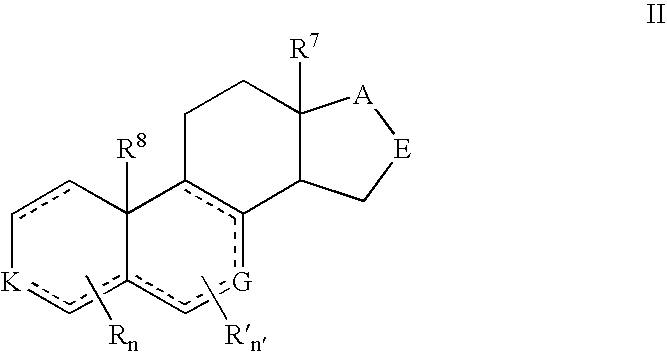
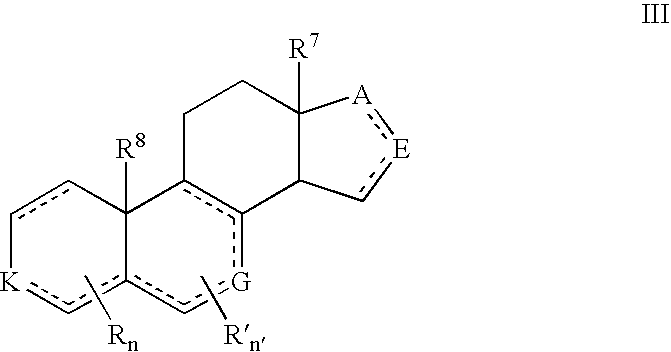
Provided herein are 19-nor C3,3-disubstituted C21-pyrazolyl steroids of Formula (I), and pharmaceutically acceptable salts thereof; wherein, R1, R2, R3a, R3b, R4a, R4b, R5, R6, and R7 are as defined herein. Such compounds are contemplated useful for the prevention and treatment of a variety of CNS-related conditions, for example, treatment of sleep disorders, mood disorders, schizophrenia spectrum disorders, convulsive disorders, disorders of memory and / or cognition, movement disorders, personality disorders, autism spectrum disorders, pain, traumatic brain injury, vascular diseases, substance abuse disorders and / or withdrawal syndromes, and tinnitus.
Owner:SAGE THERAPEUTICS
19-nor c3, 3-disubstituted c21-n-pyrazolyl steroids and methods of use thereof
ActiveUS20160108080A1Eliminate potential for oxidationImprove bioavailabilityOrganic active ingredientsSenses disorderWithdrawal syndromeSubstance abuse disorder
Provided herein are 19-nor C3,3-disubstituted C21-pyrazolyl steroids of Formula (I), and pharmaceutically acceptable salts thereof; wherein-, R1, R2, R3a, R3b, R4a, R4b, R5, R6, and R7 are as defined herein. Such compounds are contemplated useful NI for the prevention and treatment of a variety of CNS-related conditions, for example, treatment of sleep disorders, mood disorders, schizophrenia spectrum disorders, convulsive disorders, disorders of memory and / or cognition, movement disorders, personality disorders, autism spectrum disorders, pain, traumatic brain injury, vascular diseases, substance abuse disorders and / or withdrawal syndromes, and tinnitus.
Owner:SAGE THERAPEUTICS
19-NOR neuroactive steroids and methods of use thereof
ActiveUS9365611B2Eliminate potential for oxidationImprove bioavailabilityOrganic active ingredientsSenses disorderWithdrawal syndromeSubstance abuser
Provided herein are 3,3-disubstituted 19-nor-steroidal compounds according to Formula (I): and pharmaceutical compositions thereof. Such compounds are contemplated useful for the prevention and treatment of a variety of CNS-related conditions, for example, treatment of sleep disorders, mood disorders, schizophrenia spectrum disorders, disorders of memory and / or cognition, movement disorders, personality disorders, autism spectrum disorders, pain, traumatic brain injury, vascular diseases, substance abuse disorders and / or withdrawal syndromes, tinnitus, and status epilepticus.
Owner:SAGE THERAPEUTICS
19-nor neuroactive steroids and methods of use thereof
ActiveUS20160083418A1Eliminate potential for oxidationInhibit metabolismOrganic active ingredientsSenses disorderSubstance abuserWithdrawal syndrome
Provided herein are 3,3-disubstituted 19-nor-steroidal compounds according to Formula (I): and pharmaceutical compositions thereof. Such compounds are contemplated useful for the prevention and treatment of a variety of CNS-related conditions, for example, treatment of sleep disorders, mood disorders, schizophrenia spectrum disorders, disorders of memory and / or cognition, movement disorders, personality disorders, autism spectrum disorders, pain, traumatic brain injury, vascular diseases, substance abuse disorders and / or withdrawal syndromes, tinnitus, and status epilepticus.
Owner:SAGE THERAPEUTICS
19-nor C3, 3-disubstituted C21-C-bound heteroaryl steroids and methods of use thereof
ActiveUS9725481B2Eliminate potential for oxidationImprove bioavailabilityOrganic active ingredientsNervous disorderSubstance abuserWithdrawal syndrome
Provided herein are 19-nor C3,3-disubstituted steroids of Formula (I): and pharmaceutically acceptable salts thereof; wherein, , R1, R2, R3a, R3b, R4a, and R4b are as defined herein, and A is a carbon bound substituted or unsubstituted 5-to6-membered heteroaryl ring as defined herein. Such compounds are contemplated useful for the prevention and treatment of a variety of CNS-related conditions, for example, treatment of sleep disorders, mood disorders, schizophrenia spectrum disorders, convulsive disorders, disorders of memory and / or cognition, movement disorders, personality disorders, autism spectrum disorders, pain, traumatic brain injury, vascular diseases, substance abuse disorders and / or withdrawal syndromes, and tinnitus.
Owner:SAGE THERAPEUTICS
19-nor neuroactive steroids and methods of use thereof
InactiveUS20160068563A1Eliminate potential for oxidationImprove bioavailabilityNervous disorderEstrane derivativesSubstance abuserWithdrawal syndrome
Provided herein are 3,3-disubstituted 19-nor-steroidal compounds according to Formula (I): and pharmaceutical compositions thereof. Such compounds are contemplated useful for the prevention and treatment of a variety of CNS-related conditions, for example, treatment of sleep disorders, mood disorders, schizophrenia spectrum disorders, disorders of memory and / or cognition, movement disorders, personality disorders, autism spectrum disorders, pain, traumatic brain injury, vascular diseases, substance abuse disorders and / or withdrawal syndromes, tinnitus, status epilepticus.
Owner:SAGE THERAPEUTICS
Neuroactive steroids, compositions, and uses thereof
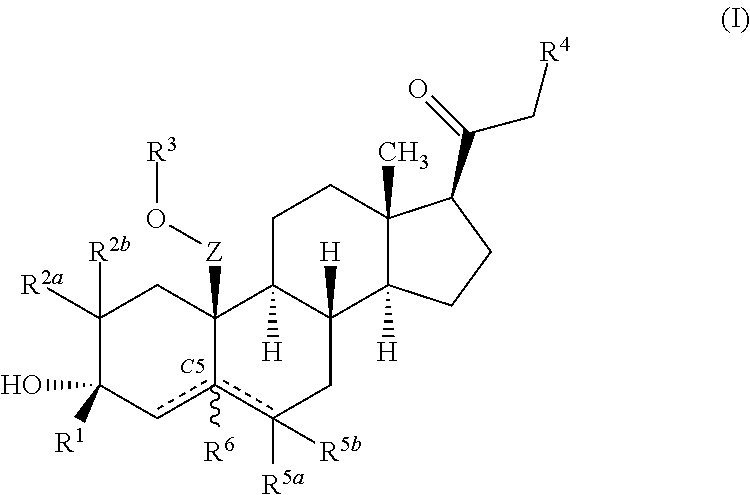
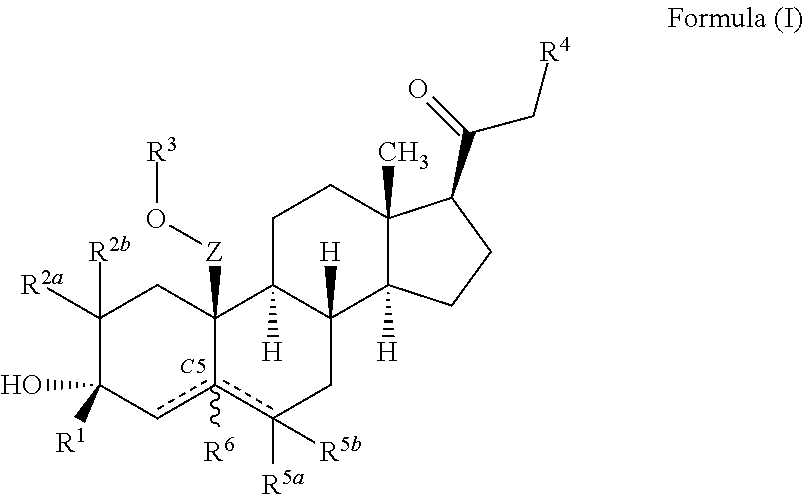
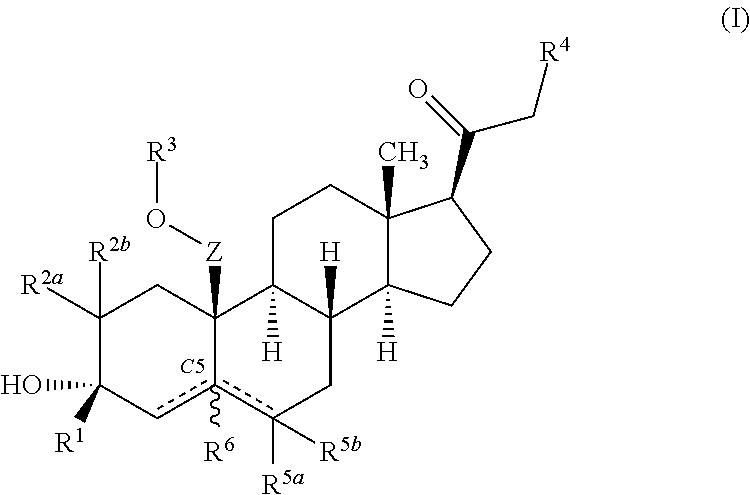
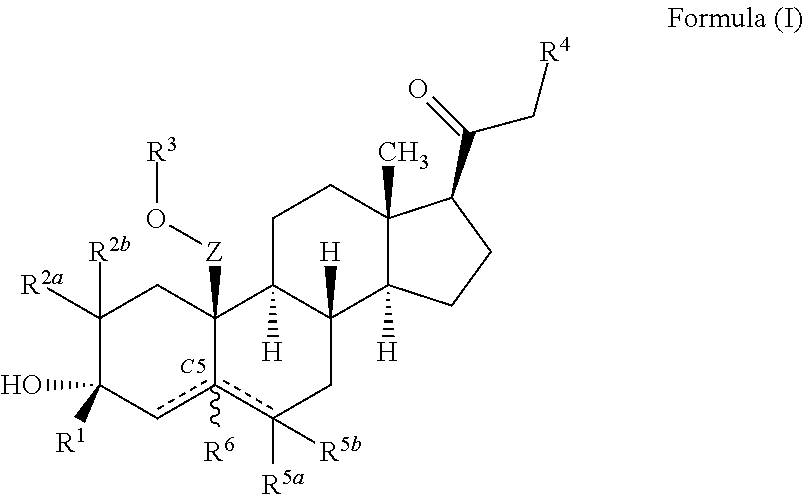
ActiveUS10246482B2Reduces avoids symptom causeGood curative effectKetal steroidsSubstance abuserWithdrawal syndrome
Described herein are steroids of Formula (I): and pharmaceutically acceptable salts thereof; wherein R1, R2a, R2b, R3, R4, R5a, R5b, R6, and Z are as defined herein. Such compounds are contemplated useful for the prevention and treatment of a variety of CNS-related conditions, for example, treatment of sleep disorders, mood disorders, schizophrenia spectrum disorders, convulsive disorders, disorders of memory and / or cognition, movement disorders, personality disorders, autism spectrum disorders, pain, traumatic brain injury, vascular diseases, substance abuse disorders and / or withdrawal syndromes, and tinnitus.
Owner:SAGE THERAPEUTICS
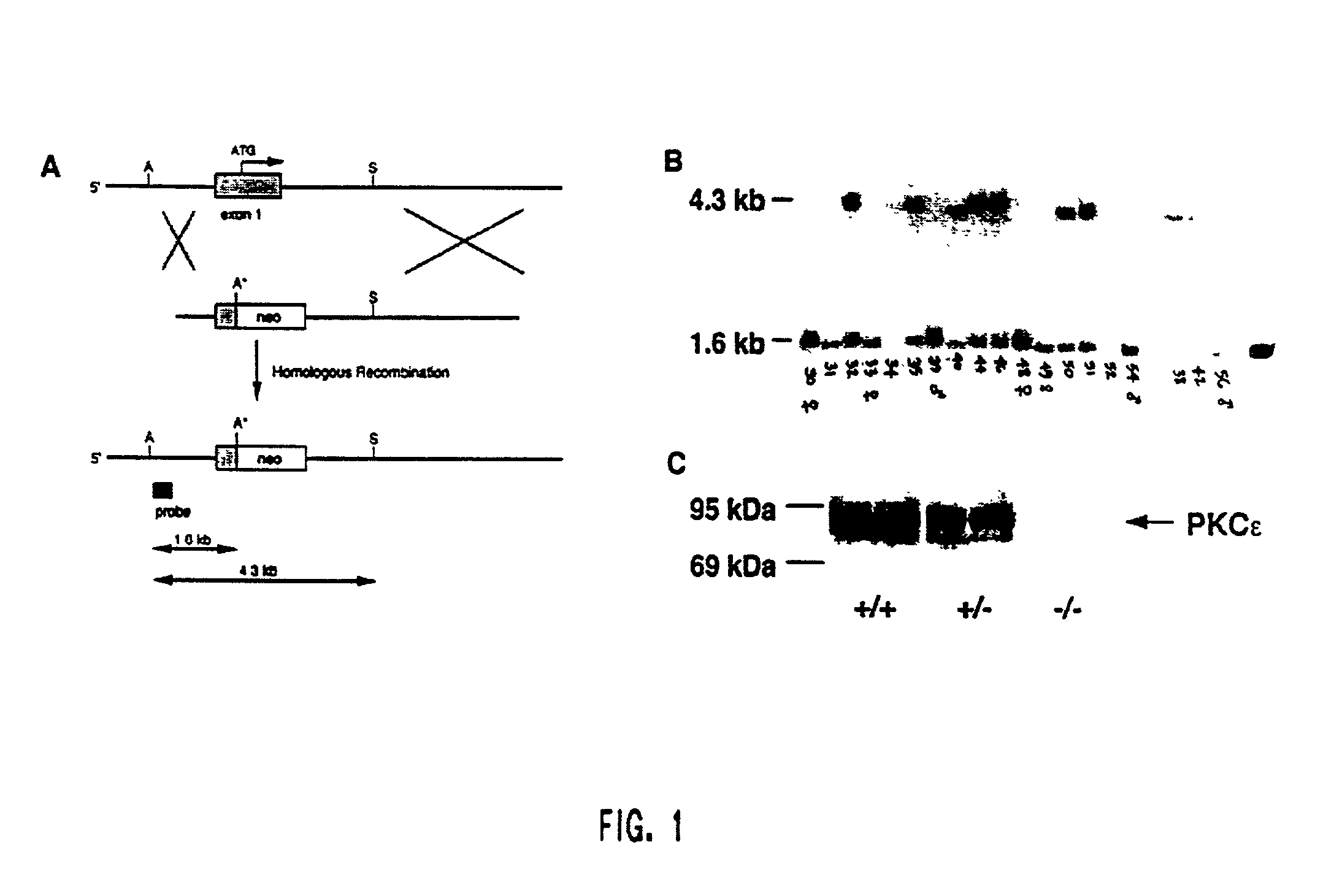
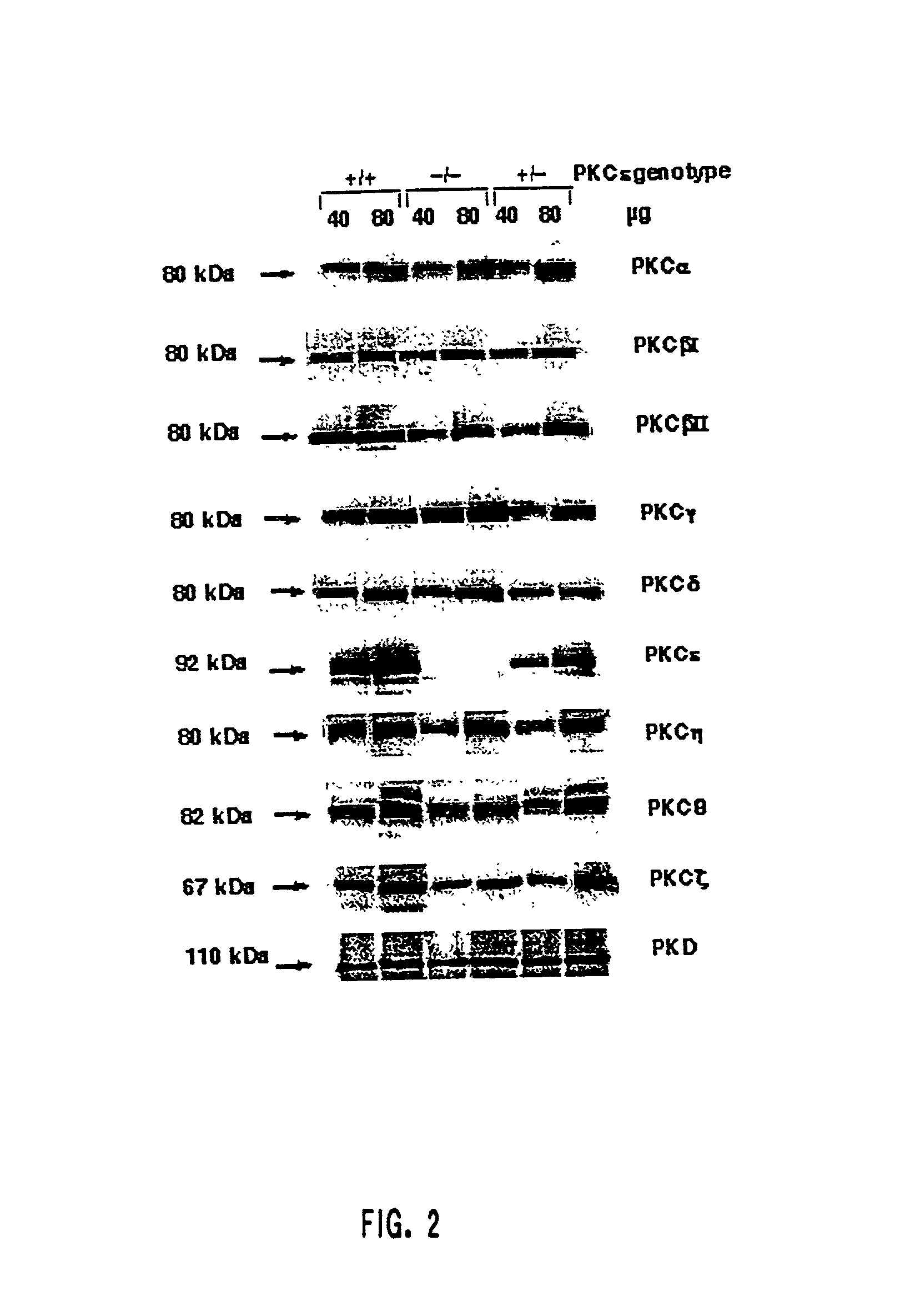
Protein kinase c epsilon as modulator of anxiety, alcohol consumption and self-administration of drugs of abuse
InactiveUS20020124272A1Nervous disorderMicrobiological testing/measurementWithdrawal syndromeAlcoholisms
The present invention is directed to the production of PKC isozyme epsi (PKCepsi)-deficient cells and non-human animals. The present invention is further directed to the identification of PKCepsi as a target for drugs that reduce anxiety. According to the present invention, PKCepsi-inhibiting compounds act in synergy with drugs acting at the GABAA receptor. The present invention is also directed to the use of modulators of PKCepsi to modulate alcohol consumption, self-administration of other drugs of abuse, and the effects of alcohol consumption as well as the use of inhibitors of PKCepsi, either alone or in conjunction with allosteric agonists of GABAA receptors, to treat conditions, such as addiction, withdrawal syndrome, skeletal muscle spasms, convulsive seizures, and epilepsy, that are amenable to treatment by allosteric agonists of GABAA receptors. Additional aspects of the present invention are diagnostic methods for identifying individuals at risk for becoming alcoholics or abusers of other drugs and kits for performing such diagnostic methods. The present invention relates to: cells and non-human animals deficient for the PKC isozyme epsi (PKCepsi); the use of PKCepsi as a target for drugs; the use of inhibitors of PKCepsi in methods of reducing anxiety and treating conditions associated with insufficient activity of the GABAA receptor; the use of modulators of PKCepsi in methods of modulating alcohol consumption, modulating self-administration of other drugs of abuse, and altering the effects of alcohol; pharmaceutical compositions comprising inhibitors of PKCepsi and allosteric agonists of GABAA receptors; and the identification of individuals with enhanced susceptibility to alcoholism or other forms of addiction.
Owner:GALLO CLINIC & RES CENT +1
19-nor c3, 3-disubstituted c21-c-bound heteroaryl steroids and methods of use thereof
ActiveUS20160083417A1Eliminate potential for oxidationInhibit metabolismOrganic active ingredientsNervous disorderDiseaseSubstance abuser
Provided herein are 19-nor C3,3-disubstituted steroids of Formula (I): and pharmaceutically acceptable salts thereof; wherein, , R1, R2, R3a, R3b, R4a, and R4b are as defined herein, and A is a carbon bound substituted or unsubstituted 5- to 6-membered heteroaryl ring as defined herein. Such compounds are contemplated useful for the prevention and treatment of a variety of CNS-related conditions, for example, treatment of sleep disorders, mood disorders, schizophrenia spectrum disorders, convulsive disorders, disorders of memory and / or cognition, movement disorders, personality disorders, autism spectrum disorders, pain, traumatic brain injury, vascular diseases, substance abuse disorders and / or withdrawal syndromes, and tinnitus.
Owner:SAGE THERAPEUTICS
Treatment of dependence withdrawal
ActiveUS20060240085A1Alleviate withdrawal symptomsBiocideNervous disorderRegimenDrug withdrawal syndrome
Dosage regimens of buprenorphine to treat withdrawal or abstinence syndrome in a drug dependent or opioid tolerant patient who is pregnant are described. The method includes treating withdrawal or abstinence syndrome of the patient by transdermal administration of an amount of buprenorphine effective to reduce withdrawal symptoms. For example, a first buprenorphine-containing transdermal dosage form for a second dosing period that is no more than about 5 days, the second dosage form comprising the same or a greater dosage of buprenorphine than the first dosage form; and a third buprenorphine-containing transdermal dosage form for a third dosing period that is at least 2 days, the third dosage form comprising the same or a greater dosage of buprenorphine than the second dosage form.
Owner:PURDUE PHARMA LP
Opioid agonist /antagonist combinations
InactiveUS20020013301A1Reducing oral abuse potential of dosage formBiocideNervous disorderOpioid AgonistOpioid antagonist
The invention is directed in part to oral dosage forms comprising a combination of an orally analgesically effective amount of an opioid agonist and an orally active opioid antagonist, the opioid antagonist being included in a ratio to the opioid agonist to provide a combination product which is analgesically effective when the combination is administered orally, but which is aversive in a physically dependent subject. Preferably, the amount of opioid antagonist included in the combination product provides at least a mildly negative, "aversive" experience in physically dependent addicts (e.g., precipitated abstinence syndrome).
Owner:PURDUE PHARMA LP
Compounds and Formulations
InactiveUS20080004250A1Organic active ingredientsKetal steroidsWithdrawal syndromeBiological condition
The instant invention provides potent antiandrogen compounds, such as 3β-acetoxyandrost-1,5-diene-17-ethylene ketal and 3β-hydroxyandrost-1,5-diene-17-ethylene ketal, and methods for their use in the prevention and treatment of biological conditions mediated by androgen receptors. Thus, for example, compounds of the invention are useful in the prevention and treatment of prostrate cancer. Furthermore, it has been discovered that compounds of the invention are useful in the prevention and treatment of androgen-independent cancers such as androgen-independent prostrate cancer. Finally, inventive compounds may be used to treat antiandrogen induced withdrawal syndrome.
Owner:NEURMEDIX +2
MIF inhibitors for treating neuropathic pain and associated syndromes
InactiveUS20070281924A1Diminishing and eliminating symptomEffective treatmentBiocideNervous disorderDrug withdrawal syndromeTherapy medication
Methods of using MIF inhibitors such as those characterized by structure I are disclosed. The MIF inhibitors can be used for treating conditions such as neuropathic pain and its associated symptoms, as well as for treating drug and behavioral addictions, such as opiate dependence. Additionally, MIF inhibitor compounds can be used for treating withdrawal syndromes after discontinuance of addictive drug use or behavior.
Owner:MEDICINOVA INC
Antagonists of RF-amide neuropeptides
InactiveUS6960574B2Good curative effectAvoid drug dependenceBiocideNervous disorderOpiate dependenceDense Core Vesicles
Disclosed are compounds having the formula: whereR1=H, C1-C6 alkyl, cycloalkyl, R2=H, C1-C6 alkyl, cycloalkylW=CnH2n-m—NH (n=1-6, m=0, 2, or 4), X=R3=Y=Z=CONR8(CH2)n, CONR8(CH2)nCO, P(CH3)OCHR8OCOR9, SO2, SO2(CH2)n, SO2(CH2)nCO, SO2NR8(CH2)n, SO2NR8(CH2)nCO, n=1-4R4=H, (CH2)nOH, (CH2)nOCOR10, (CH2)nNR10R11, (CH2)nCONR10R11, n=0-4R5=H, (CH2)nNR12R13, n=0-4R6=H, (CH2)nNR14R15, n=0-4R7=H, C1-C6 alkyl, cycloalkyl; R8=H, C1-C6 alkyl, cycloalkyl; R9=H, C1-C6 alkyl, cycloalkyl;R10=H, C1-C6 alkyl, cycloalkyl; R11=H, C1-C6 alkyl, cycloalkyl; R12=H, C1-C6 alkyl, cycloalkyl;R13=H, C1-C6 alkyl, cycloalkyl; R14=H, C1-C6 alkyl, cycloalkyl; R15=H, C1-C6 alkyl, cycloalkyl.Dashed lines: optional; conformational constraint by (CH2)n, n=1-3, R′=H or O(═) as well as pharmaceuticals compositions and methods for the treatment of opiate addiction, opiate dependence, opiate tolerance, opiate related abstinence syndrome, nicotine addition and obesity based thereon.
Owner:UNIV OF FLORIDA RES FOUNDATION INC
19-nor neuroactive steroids and methods of use thereof
ActiveUS20170233432A1Eliminate potential for oxidationImprove bioavailabilityNervous disorderEstrane derivativesWithdrawal syndromeSubstance abuser
Owner:SAGE THERAPEUTICS
Prostate Cancer Treatment
InactiveUS20080009472A1Organic active ingredientsKetal steroidsWithdrawal syndromeBiological condition
The instant invention provides potent antiandrogen compounds, such as 3β-acetoxyandrost-1,5-diene-17-ethylene ketal and 3β-hydroxyandrost-1,5-diene-17-ethylene ketal, and methods for their use in the prevention and treatment of biological conditions mediated by androgen receptors. Thus, for example, compounds of the invention are useful in the prevention and treatment of prostrate cancer. Furthermore, it has been discovered that compounds of the invention are useful in the prevention and treatment of androgen-independent cancers such as androgen-independent prostrate cancer. Finally, inventive compounds may be used to treat antiandrogen induced withdrawal syndrome.
Owner:NEURMEDIX +2
Neuroactive steroids, compositions, and uses thereof
ActiveUS20170247406A1Reduces avoids symptom causeGood curative effectKetal steroidsSubstance abuserWithdrawal syndrome
Described herein are steroids of Formula (I): and pharmaceutically acceptable salts thereof; wherein R1, R2a, R2b, R3, R4, R5a, R5b, R6, and Z are as defined herein. Such compounds are contemplated useful for the prevention and treatment of a variety of CNS-related conditions, for example, treatment of sleep disorders, mood disorders, schizophrenia spectrum disorders, convulsive disorders, disorders of memory and / or cognition, movement disorders, personality disorders, autism spectrum disorders, pain, traumatic brain injury, vascular diseases, substance abuse disorders and / or withdrawal syndromes, and tinnitus.
Owner:SAGE THERAPEUTICS
Neuroactive steroids, compositions, and uses thereof
PendingUS20180179247A1Eliminate potential for oxidationInhibit metabolismOrganic active ingredientsNervous disorderWithdrawal syndromeSubstance abuse disorder
Provided herein are 19-nor C3, 3-disubstituted steroids of Formula (I): and pharmaceutically acceptable salts thereof; wherein R1, R2, R3, and R4 are as defined herein, and A is a heteroaryl ring system comprising 3 or 4 nitrogens as defined herein. Such compounds are contemplated useful for the prevention and treatment of a variety of CNS-related conditions, for example, treatment of sleep disorders, mood disorders, schizophrenia spectrum disorders, convulsive disorders, disorders of memory and / or cognition, movement disorders, personality disorders, autism spectrum disorders, pain, traumatic brain injury, vascular diseases, substance abuse disorders and / or withdrawal syndromes, and tinnitus.
Owner:SAGE THERAPEUTICS
N-But-3-Enyl norbuprenophine and its use as analgesic
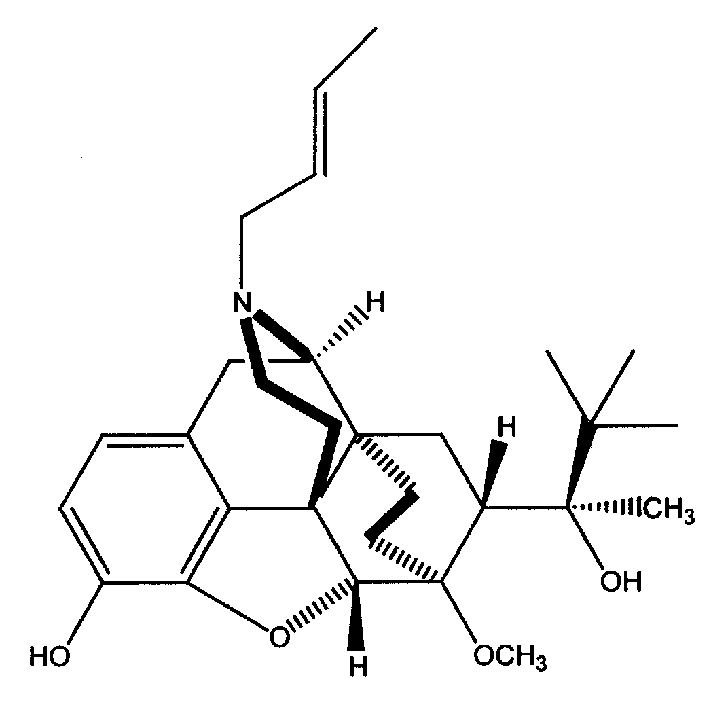
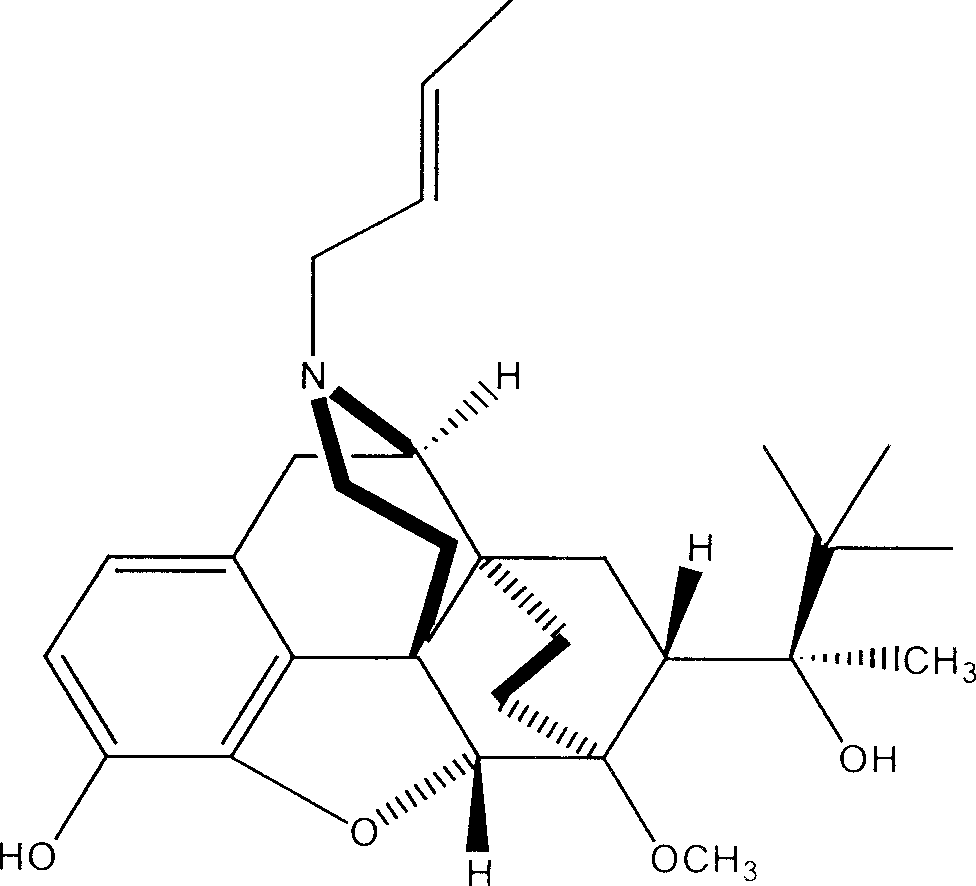
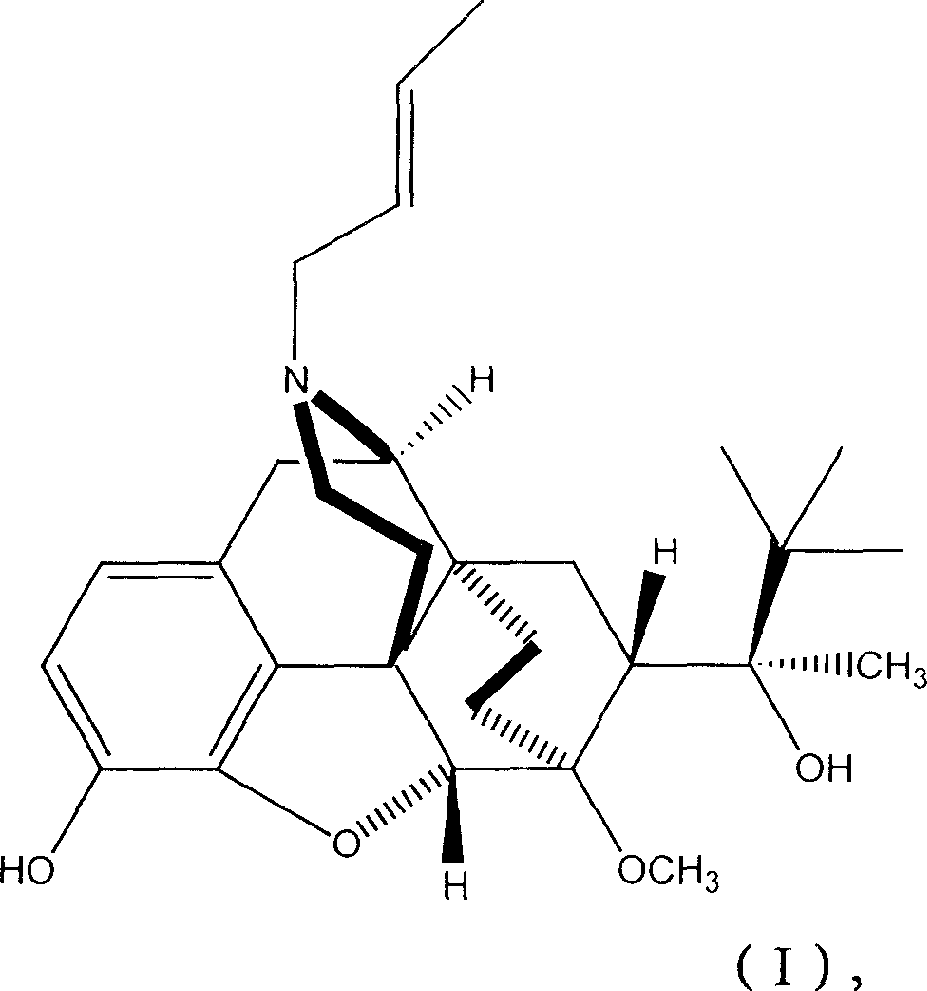
The present invention provides compound having the structure and pharmaceutically acceptable salts or derivatives thereof, as well as compositions including such compounds. The invention also provides methods of (1) preventing pain, (2) treating pain, (3) inducing sedation, (4) treating opiate addiction, (5) treating opiate withdrawal (abstinence syndrome) and / or (6) treating cough in a patient in need thereof by administering a compound or composition of the invention.
Owner:EURO-CELTIQUE SA
Monoamine oxidase (MAO) inhibitors and uses thereof
InactiveUS20050176777A1Inhibiting MAO activityImprove concentrationBiocideNervous disorderSubstance abuserWithdrawal syndrome
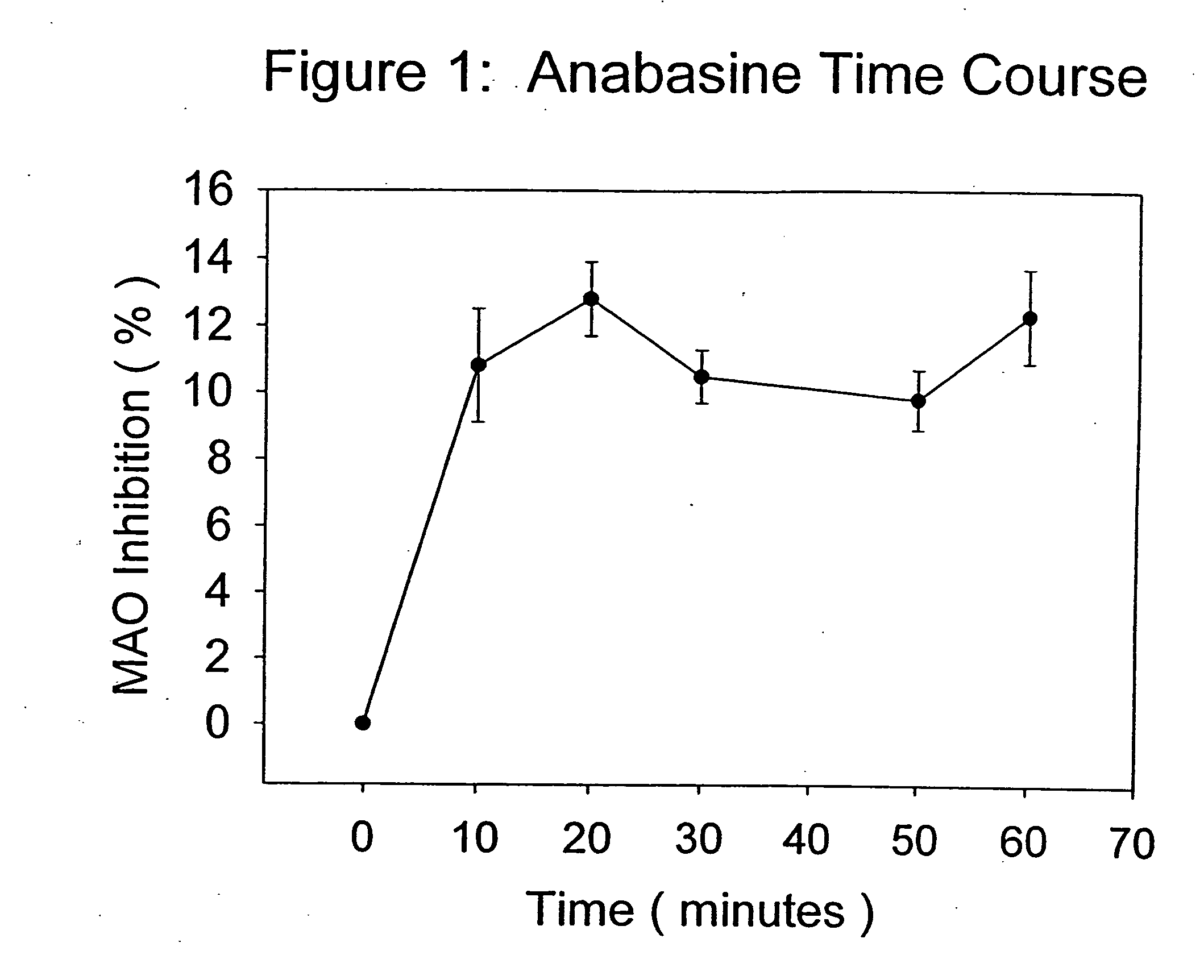
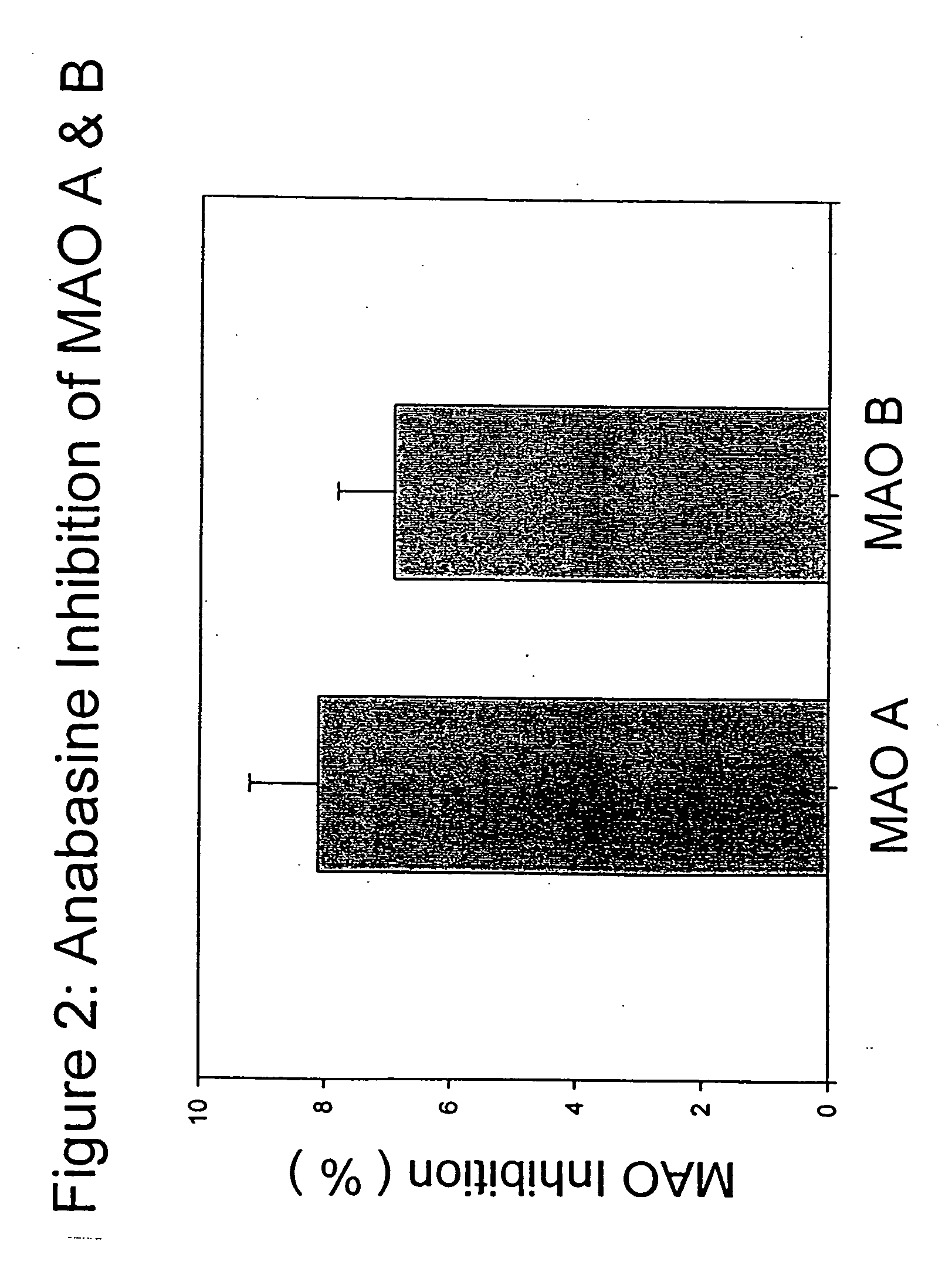
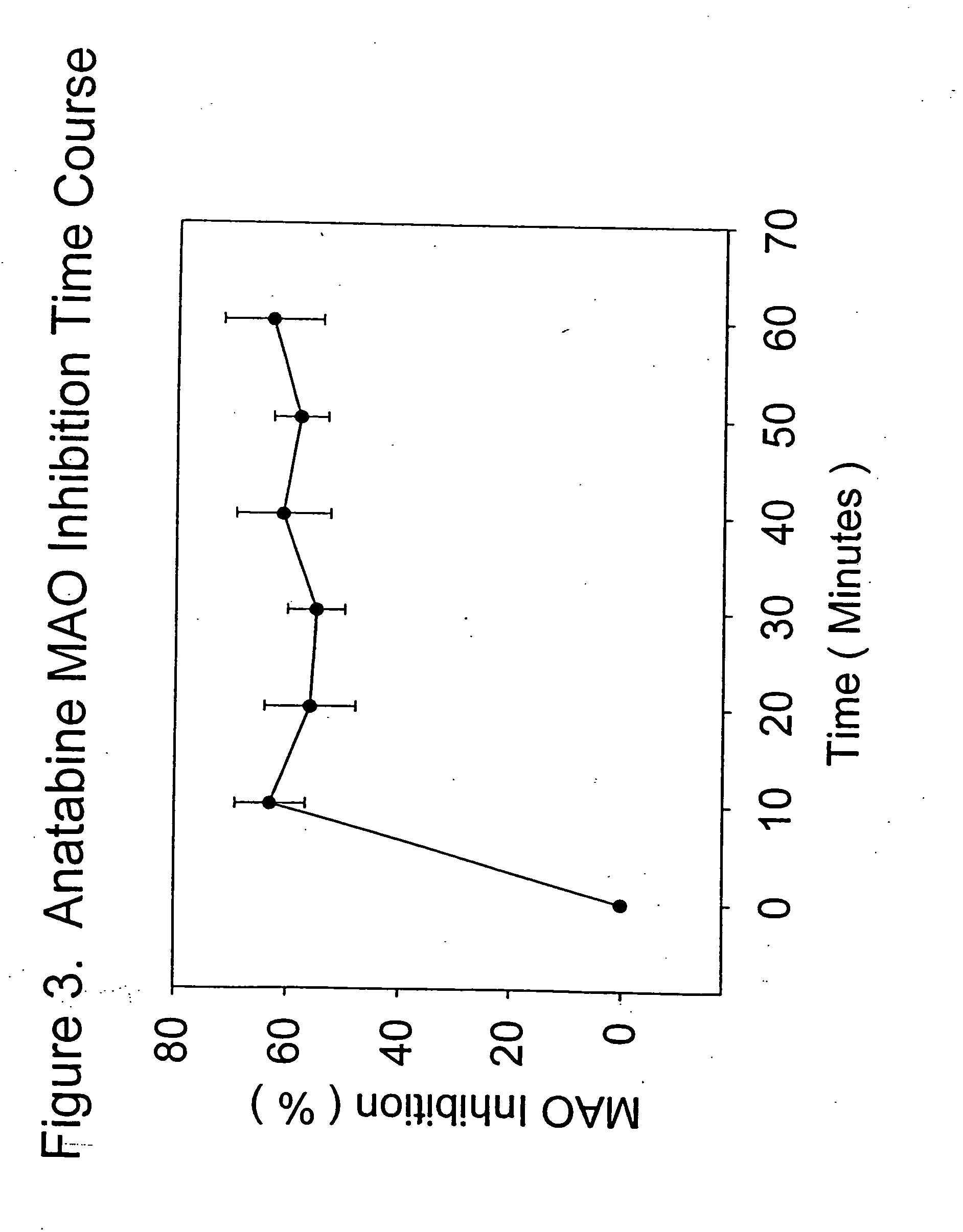
The present invention provides a group of tobacco alkaloids, tobacco extract, Yerbamaté extract, and an extract of chewing gum and lozenges which are modulators of monoamine oxidase (MAO) activity (i.e., compounds and substances which inhibit MAO enzyme and prevent its biological activity). The MAO inhibitors of the present invention can cause an increase in the level of norepinephrine, dopamine, and serotonin in the brain and other tissues, and thus can cause a wide variety of pharmacological effects mediated by their effects on these compounds. The MAO inhibitors of the present invention are useful for a variety of therapeutic applications, such as the treatment of depression, disorders of attention and focus, mood and emotional disorders, Parkinson's disease, extrapyramidal disorders, hypertension, substance abuse, smoking substitution, anti-depression therapy, eating disorders, withdrawal syndromes, and the cessation of smoking.
Owner:REGENT COURT TECH
Substance exhibiting antidepressant property
InactiveUS20060287383A1Significant antidepressant effectEffect andBiocideNervous disorderWithdrawal syndrome2-Pyrrolidone
The invention relates to medicine, more specifically to antidepressant pharmacology which selectively improves a diminished mood dominated by asthenic emotions. The inventive antidepressant substance is embodied in the form of N-carbamoyl-methyl-4-phenyl-2-pyrrolidone. Said invention increases the evidence of antidepressant effect after a single dose, prevents sedative and cholinergic effects, avoids the development of a tolerance and withdrawal syndrome during a course of application and allows for the unrestricted use of said substance for professional activity.
Owner:AKHAPKINA VALENTINA IVANOVNA +5
Method for the treatment of cancer using phosphatidic acid-comprising compositions
InactiveUS6358937B1Improve liquidityEffective treatmentBiocideNervous disorderWithdrawal syndromeDisease
A lipid preparation comprising at least about 10% phosphatidic acid (PA) is used to treat human conditions or disease. Examples of such conditions or disease are withdrawal syndromes or cancer.
Owner:MODUS BIOLOGICAL MEMBRANES
Pharmaceutical formulations comprising opioid receptor agonist as active ingredients, methods of manufacture and therapeutic uses thereof
ActiveUS20190117556A1Formula SafetyAdequate shelf lifeOrganic active ingredientsNervous disorderBULK ACTIVE INGREDIENTDrug withdrawal syndrome
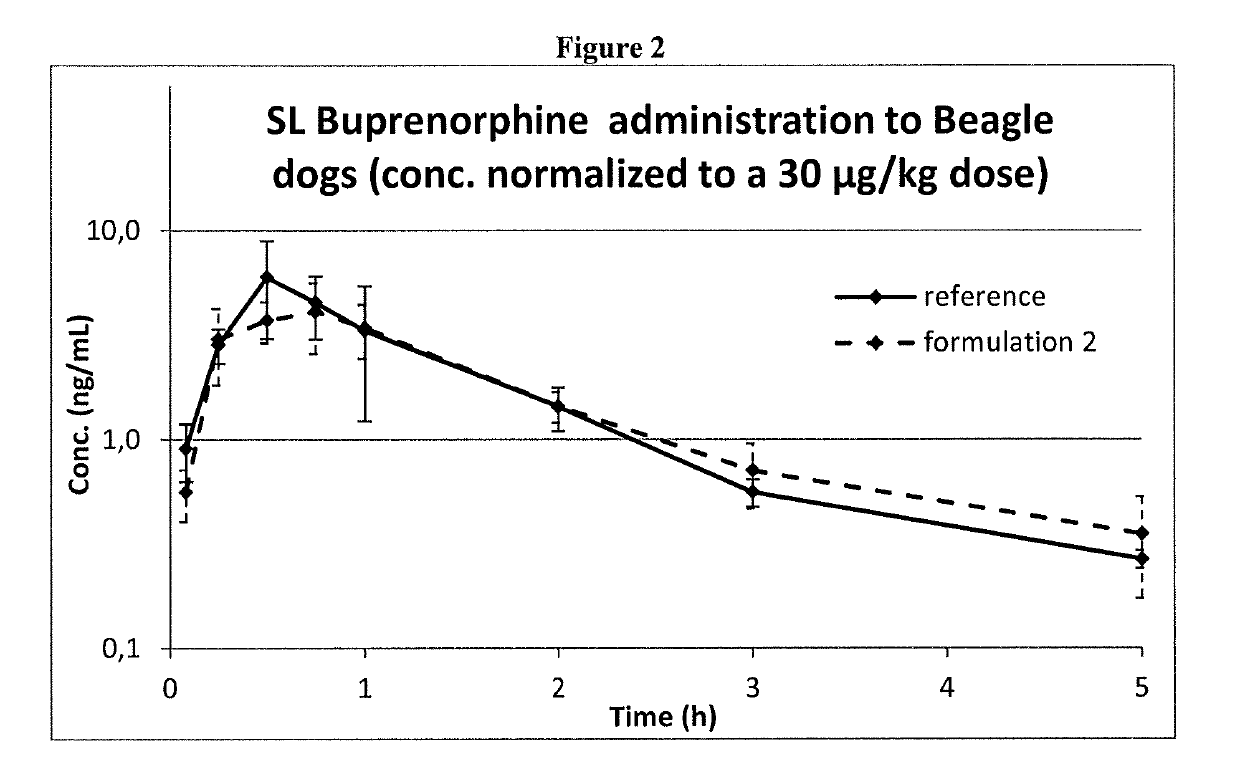
Formulation comprising buprenorphine or a pharmaceutically acceptable salt thereof as the sole active ingredient, a viscosity enhancer, and a buffering agent in an amount to provide a pH of from 5.0 to 7.0 are useful for treating opioid withdrawal syndrome.
Owner:CHIESI FARM SPA
Novel biological method for detoxification
The invention belongs to the biological gene engineering field, relating to a production method of recombined adenovirus expressing endogenous opioid peptides (Beta-endorphin) and having curative effect to withdrawal syndrome, and a method for curing the withdrawal syndrome by utilizing the recombined adenovirus. The methods can also be used in the gene therapy of other diseases.
Owner:俞卫锋 +1
Freeze-dried reparation of tetrodotoxin and the producing method thereof
InactiveUS20110201627A1Water contentIncrease moistureOrganic active ingredientsNervous disorderOpiateMannitol
A stable freeze-dried powder preparation of tetrodotoxin and the producing method thereof. The freeze-dried powder preparation has tetrodotoxin as the main active ingredient, and comprises solubilizer, excipient and stabilizer. The said solubilizer is citric acid. The excipient is sodium chloride, mannitol or their composite. The stabilizer is dextran, trehalose or their composite. The ratio of tetrodotoxin, excipient and stabilizer is 1:150-3000:50-500 or 50-6000. Preferably, the preparation comprises lidocaine hydrochloride as function modulator. The preparation of the present invention can be used for avoiding the dependent abstinence syndrome of drugs such as opiates and cannabis.
Owner:XIAMEN ZHAOYANG BIOLOGICAL ENG +1
Drug giving-up medicine made-up by non-thebaica medicines
ActiveCN1579551ANon-addictiveThe formula is concise and effectiveSalicyclic acid active ingredientsNervous disorderAdditive ingredientDrug withdrawal syndrome
The invention relates to a compound medicine, especially a compound medicine made up of nonopium medicine with biological abstinence of drugs function for curing or releasing 'abstinence syndrome'. The product is mainly made up of flowing ingredients with following weigh proportion: nonopium medicine pain easing medicine 10-80%, choline resisting medicine 0.0001-5%, psychosis resisting medicine 10-80%. The product contains no opium medicine, no addict; it belongs to non-substitute type medicine. It can control the bad symptom when in 'abstinence of drugs'.
Owner:尤伟昶 +2
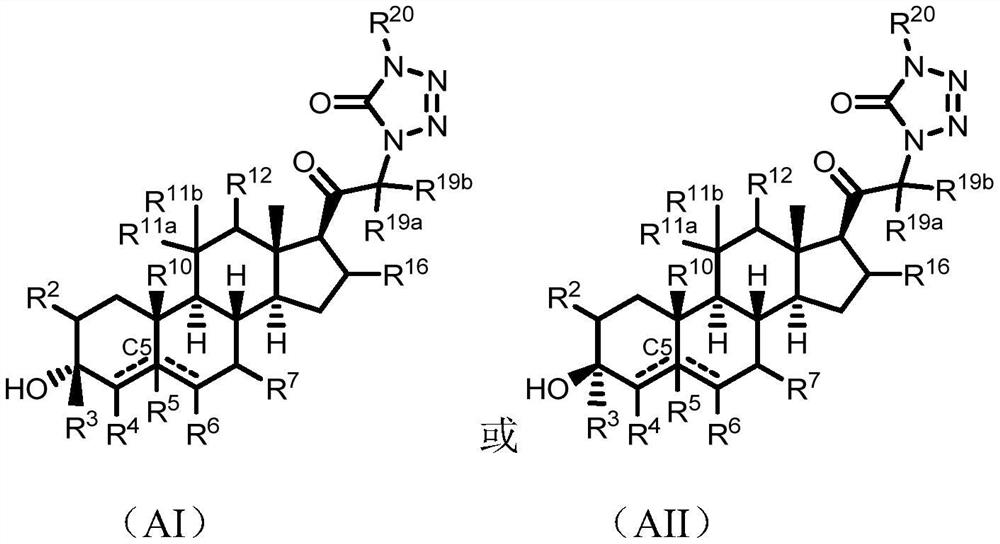
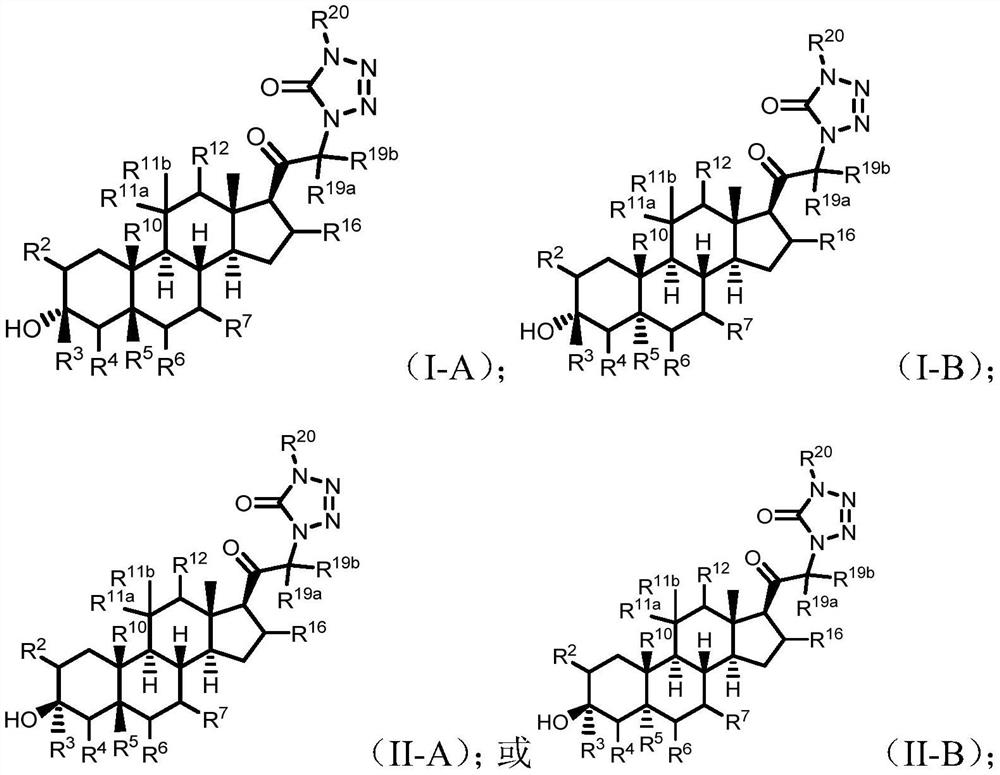
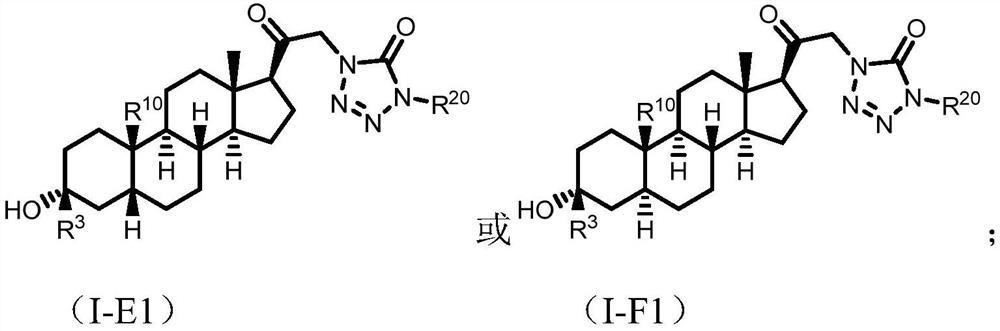
Tetrazolone substituted steroids and use thereof
The present disclosure relates to compounds of formula (AI), (I), (AII), and (II), or a pharmaceutically acceptable salt, solvate, stereoisomer, or tautomer thereof, a pharmaceutical composition comprising a compound of formula (AI), (I), (AII), and (II), and use thereof, wherein R2, R3, R4, R5, R6, R7, R10, R11a, R11b, R12, R16, R19a, R19b, and R20 are described herein. Such compounds are envisioned useful for the prevention and treatment of a variety of CNS-related conditions, for example, treatment of sleep disorders, mood disorders, movement disorders, convulsive disorders, schizophrenin spectrum disorders, disorders of memory and / or cognition, personality disorders, autism spectrum disorders, pain, traumatic brain injury, vascular diseases, substance abuse disorders and / or withdrawal syndromes, or tinnitus etc.
Owner:BEIJING XUANYI PHARMASCIENCES CO LTD
Method for treating neonatal opiod withdrawal syndrome
InactiveUS20200330455A1Stable and fastReduce usageOrganic active ingredientsNervous disorderMedicineDrug withdrawal syndrome
Owner:CHIESI FARM SPA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com