Monoamine oxidase (MAO) inhibitors and uses thereof
a monoamine oxidase and inhibitor technology, applied in the field of monoamine oxidase inhibitors, can solve problems such as adverse drug-food interactions, and achieve the effect of inhibiting mao activity and increasing the concentration of monoamine compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
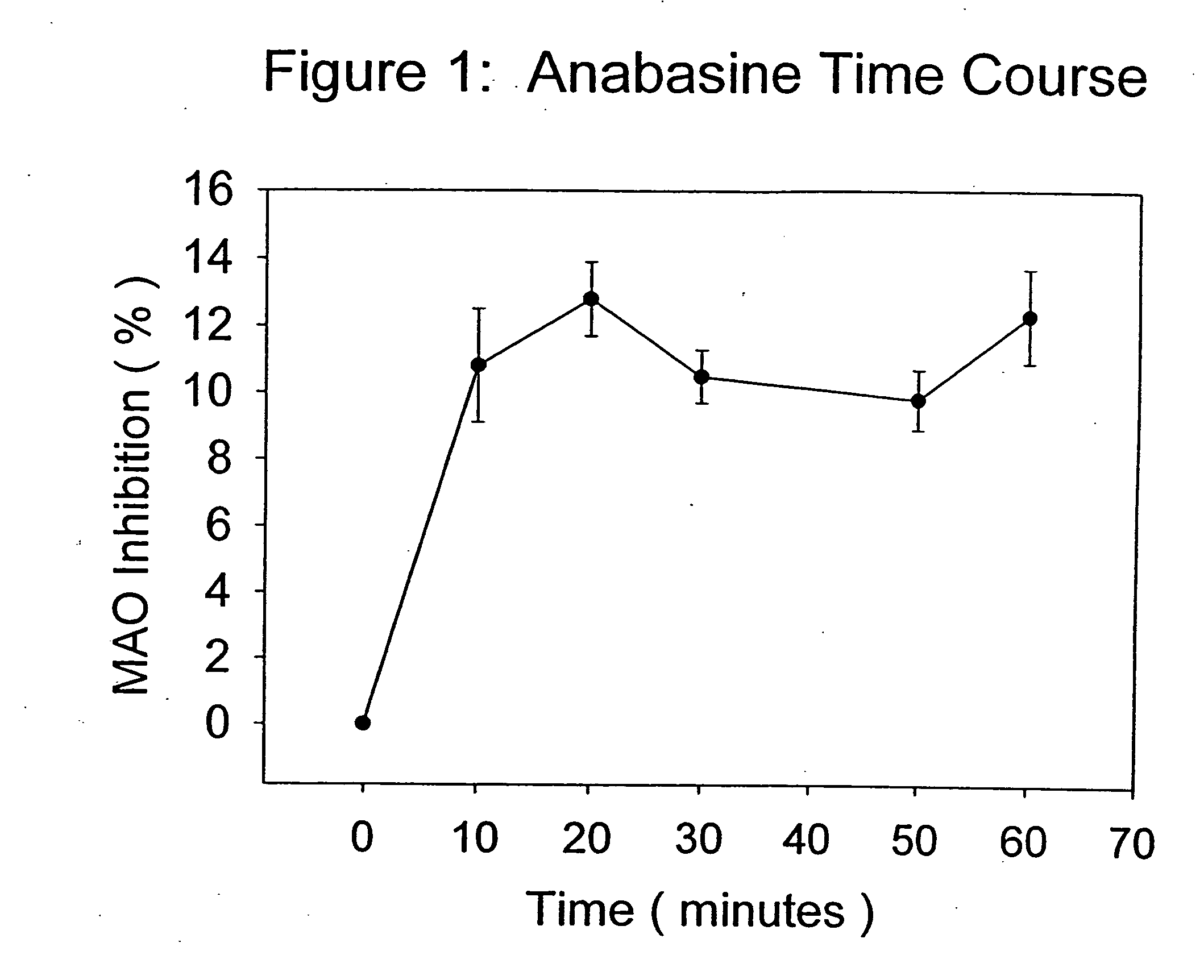
[0058] Anabasine, in its purified form, was dissolved in distilled water in a maximal inhibition concentration of 0.2 mg / ml, and tested according to the procedure described above. At maximal or saturating inhibition concentrations, anabasine was effective at inhibiting MAO activity by approximately 10-13%, and was effective at inhibiting the enzyme at all time points in the reaction.
[0059]FIG. 1 presents the means (plus or minus the standard errors of the means) for the percent inhibition of MAO activity produced by saturating concentrations of anabasine over 60 minutes of MAO activity measured as described above. Each data point represented the mean of 5 determinations. All the data points shown in FIG. 1 were statistically, significantly different from the sham control at each time point tested (student t test, p<0.01), and were representative of multiple experiments.
[0060] Since anabasine was an inhibitor of MAO activity, further studies were conducted to evaluate if this agent...
example 2
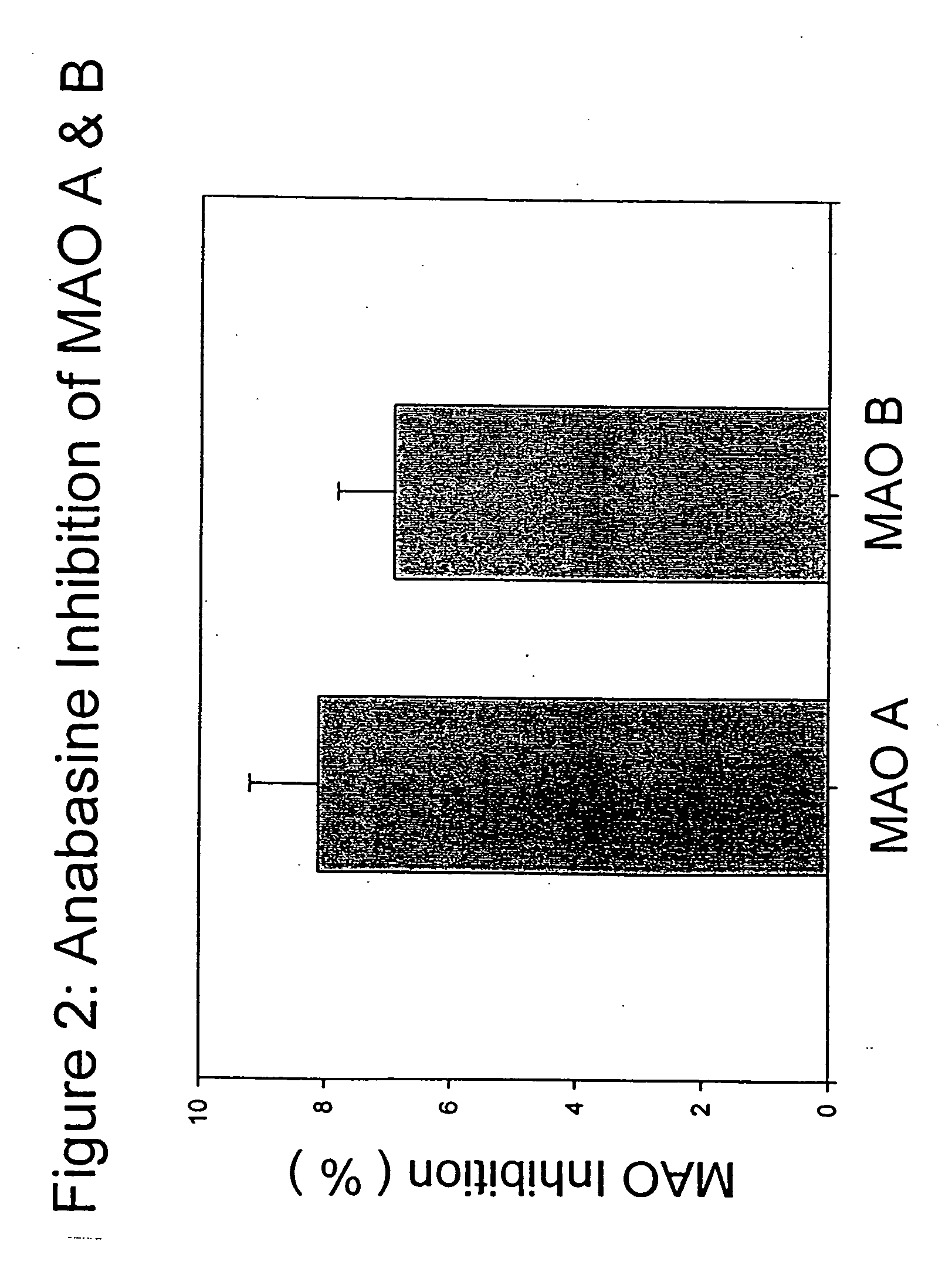
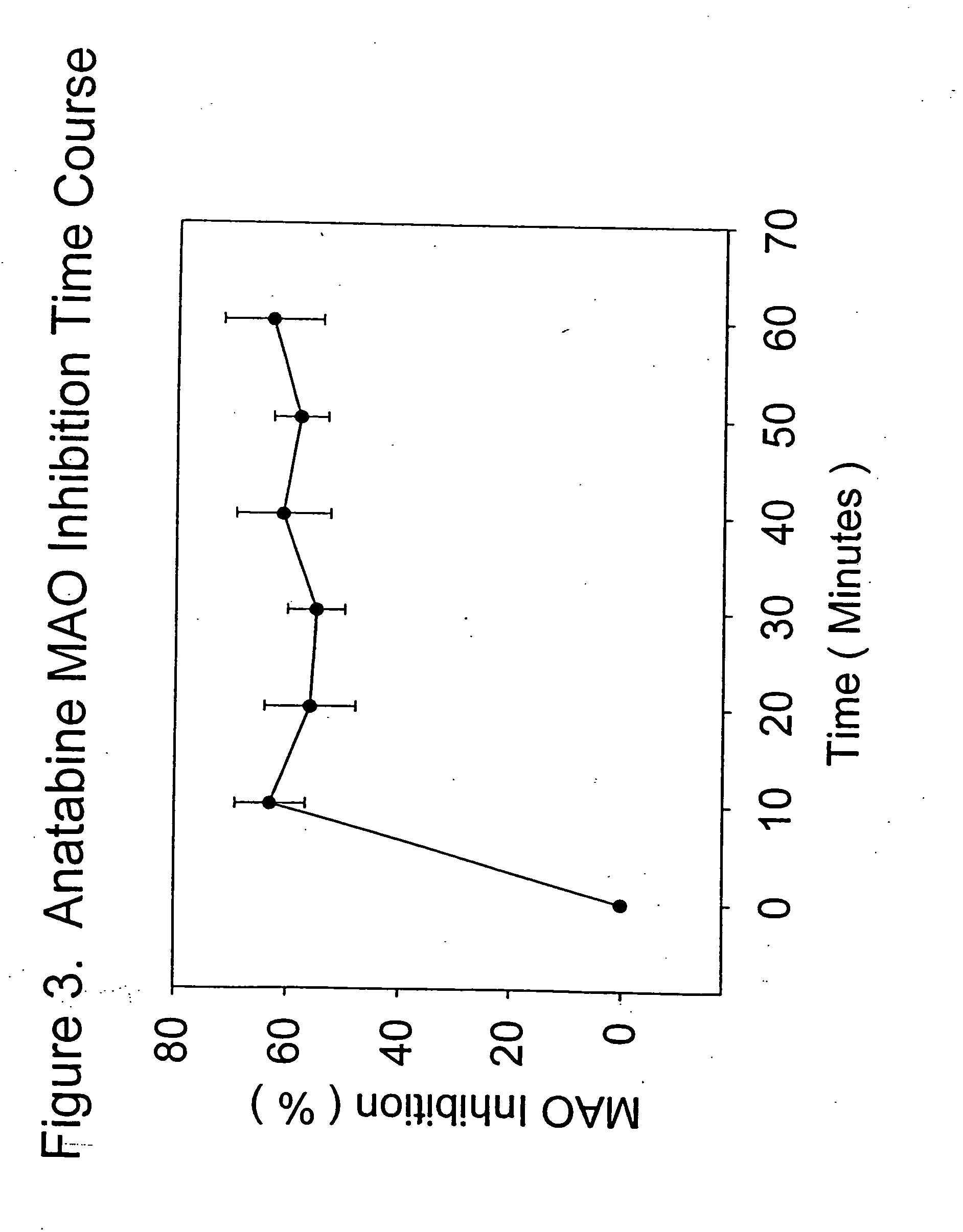
[0061] Anatabine in its purified form, was dissolved in distilled water in a maximal inhibition concentration of 0.1 mg / ml, and tested according to the procedure described above. At maximal or saturating inhibition concentrations, anatabine was effective at inhibiting MAO activity by approximately 60%. This result shows that anatabine may be much safer as a medication than standard MAO enzyme inhibitors. Anatabine was effective at inhibiting the enzyme at all time points in the reaction, and was equally effective in inhibiting both MAO A and MAO B activities.
[0062]FIG. 3 presents the means (plus or minus the standard errors of the means) for the percent inhibition of MAO activity produced by saturating concentrations of anatabine over 60 minutes of MAO activity measured as described above. Each data point represented the mean of 6 determinations. Anatabine was an effective MAO inhibitor at maximal concentrations, inhibiting the enzyme by approximately 60%, as discussed above. All t...
example 3
[0064] Nornicotine in its purified form, was dissolved in distilled water in a maximal inhibition concentration of 0.08 mg / ml, and tested according to the procedure described above. At maximal or saturating inhibition concentrations, nornicotine was effective at inhibiting MAO activity by approximately 80 to 95%, and was effective at inhibiting the enzyme at all time points in the reaction. Nornicotine was also equally effective in inhibiting both MAO A and MAO B activities.
[0065]FIG. 5 presents the means (plus or minus the standard errors of the means) for the percent inhibition of MAO activity produced by saturating concentrations of nornicotine over 60 minutes of MAO activity measured as described above. Each data point represented the mean of 6 determinations. Nomicotine was an effective MAO inhibitor at maximal concentrations, inhibiting the enzyme by approximately 80-95%, as discussed above. All the data points shown in FIG. 5 were statistically, significantly different from ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| concentration pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com