Transdermal delivery system comprising buprenorphine
a technology of buprenorphine and transdermal therapy, which is applied in the direction of biocide, drug composition, bandages, etc., can solve problems such as the potential to be subject to illicit us
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
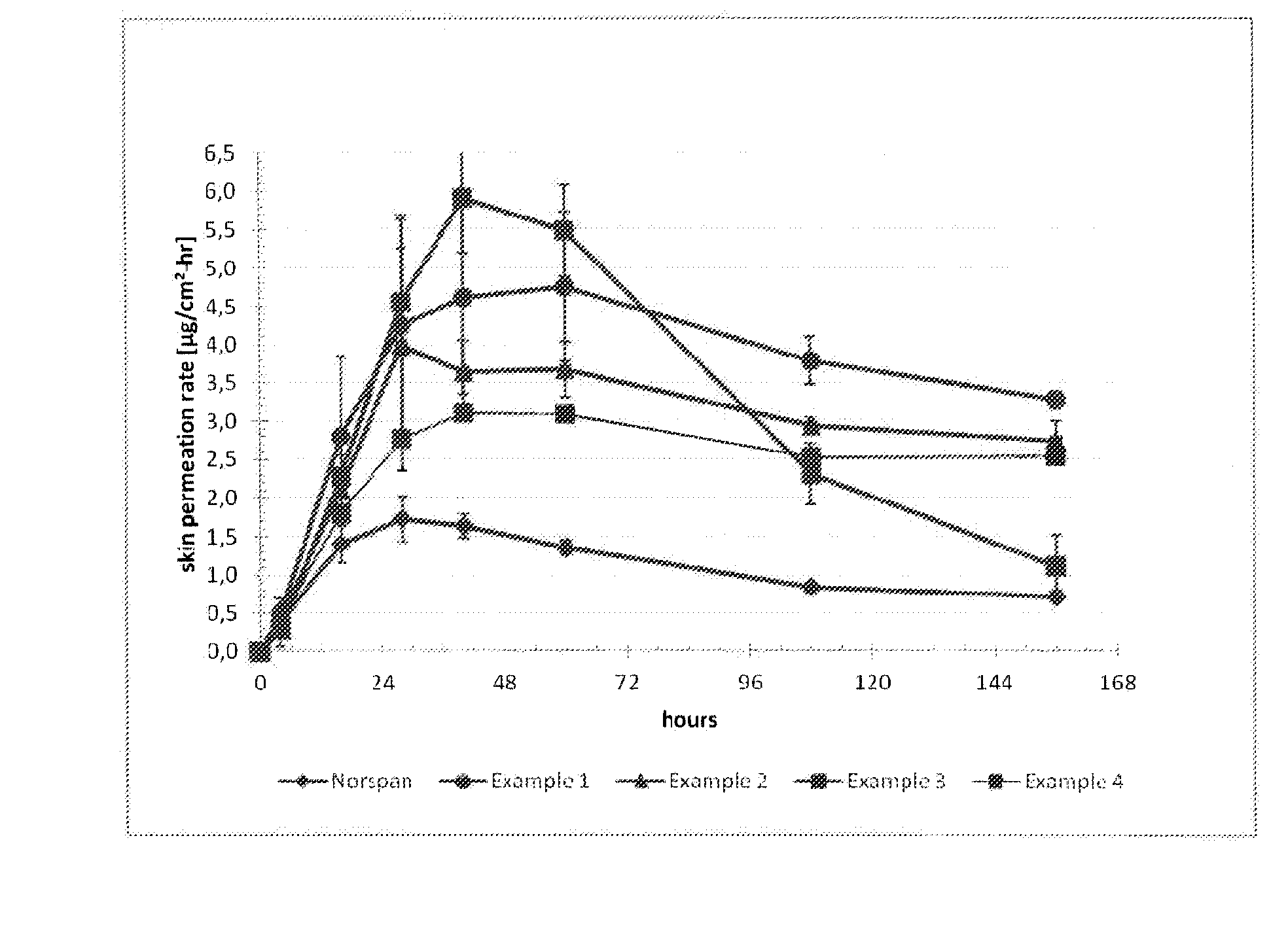
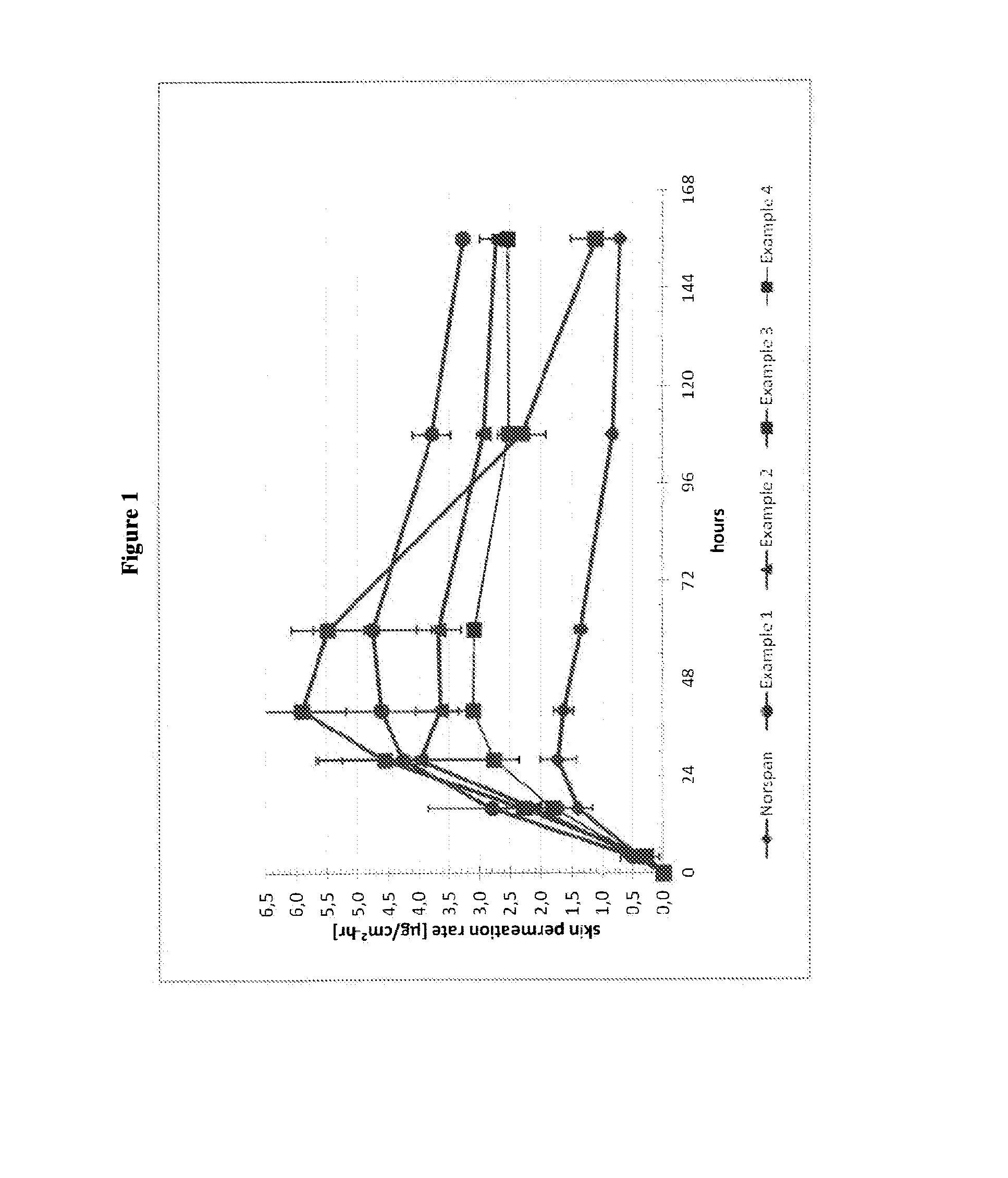
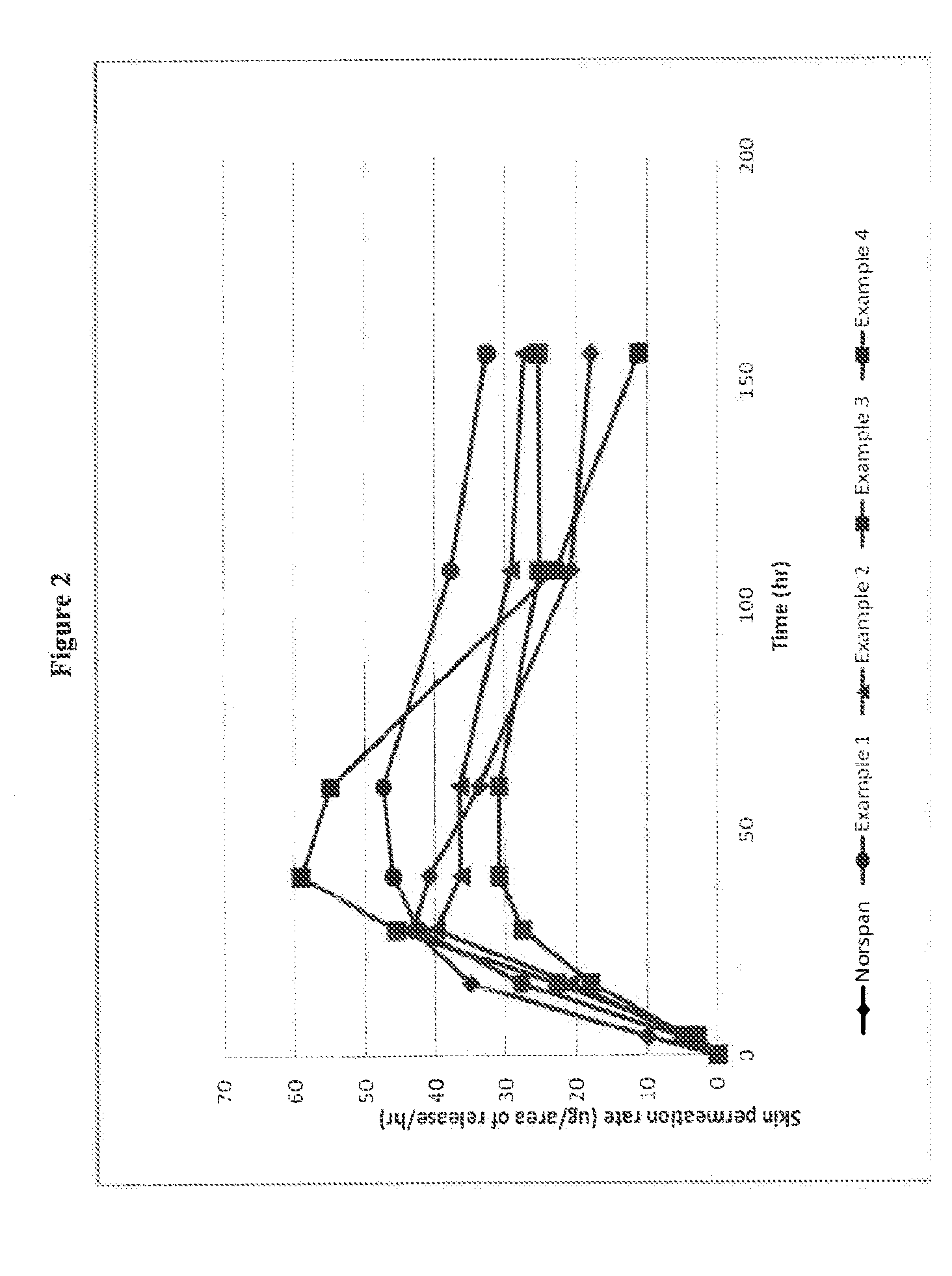
Image
Examples
example 1
[0213]The composition of the buprenorphine base-containing adhesive solution is summarized in Table 1 below.
TABLE 1Amt / unitIngredient (Trade Name)(kg)Buprenorphine base3.65Levulinic acid3.65Ethanol1.97Polysiloxane adhesive in n-heptane40.0Solids content of 73% by weight(BIO-PSA 7-4301 from Dow CorningHealthcare)n-heptane2.87Total52.14
[0214]In a stainless steel vessel, 3.65 kg of buprenorphine were suspended in 3.65 kg of levulinic acid and 1.97 kg of ethanol. With stirring, 40.0 kg of a polysiloxane adhesive in the form of a solution in n-heptane having a solids content of 73% by weight and 2.87 kg of heptane were added. The mixture was stirred until the buprenorphine base was fully dissolved, to give 52.14 kg of a buprenorphine-containing adhesive solution with 7% of buprenorphine, with a solids content of 70% (buprenorphine base-containing adhesive solution).
[0215]The buprenorphine base-containing adhesive solution was coated on an adhesive polyethylene terephthalate film (e.g., S...
example 2
[0217]The composition of the buprenorphine base-containing adhesive solution is summarized in Table 2 below.
TABLE 2Amt / unitIngredient (Trade Name)(kg)Buprenorphine base3.65Levulinic acid2.56Ethanol1.83Polysiloxane adhesive in n-heptane41.49Solids content of 73% by weight(BIO-PSA 7-4301 from Dow CorningHealthcare)n-heptane2.61Total52.14
[0218]The process of manufacture was as described for Example 1. The coating thickness was also chosen such that removal of the solvents results in a coating weight of the matrix layer of 120 g / m2 and thus resulted in 10% by weight buprenorphine base and 7% by weight levulinic acid in this matrix layer.
example 3
[0219]The composition of the buprenorphine base-containing adhesive solution is summarized in Table 3 below.
TABLE 3Amt / unitIngredient (Trade Name)(kg)Buprenorphine base3.65Levulinic acid3.65Ethanol1.97Polysiloxane adhesive in n-heptane39.46Solids content of 74% by weight(BIO-PSA 7-4201 from Dow CorningHealthcare)n-heptane3.41Total52.14
[0220]The process of manufacture was as described for Example 1. The coating thickness was also chosen such that removal of the solvents results in a coating weight of the matrix layer of 120 g / m2 and thus resulted in 10% by weight buprenorphine base and 10% by weight levulinic acid in this matrix layer.
PUM
| Property | Measurement | Unit |
|---|---|---|
| area | aaaaa | aaaaa |
| area | aaaaa | aaaaa |
| area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com