Novel buprenorphine ester derivatives and process for preparing the same and medical compounds with long-acting analgesic effect
A technology of buprenorphine monocarboxylate and dibuprenorphine dicarboxylate, applied in the field of novel buprenorphine ester derivatives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0117] The following examples are provided for illustrative purposes only, and are not intended to limit the scope of the present invention.
[0118] Table 1 below shows the chemical structures of preferred buprenorphine ester derivatives according to the present invention.
[0119] Table 1. Molecular structures of buprenorphine HCl, buprenorphine base and ester derivatives according to the present invention
[0120]
[0121] Bup: stands for buprenorphine
[0122] The buprenorphine ester derivatives listed in Table 1 can be synthesized by suitable known methods, such as those disclosed in US Pat. Nos. 5,750,534 and 6,225,321.
Synthetic example 1
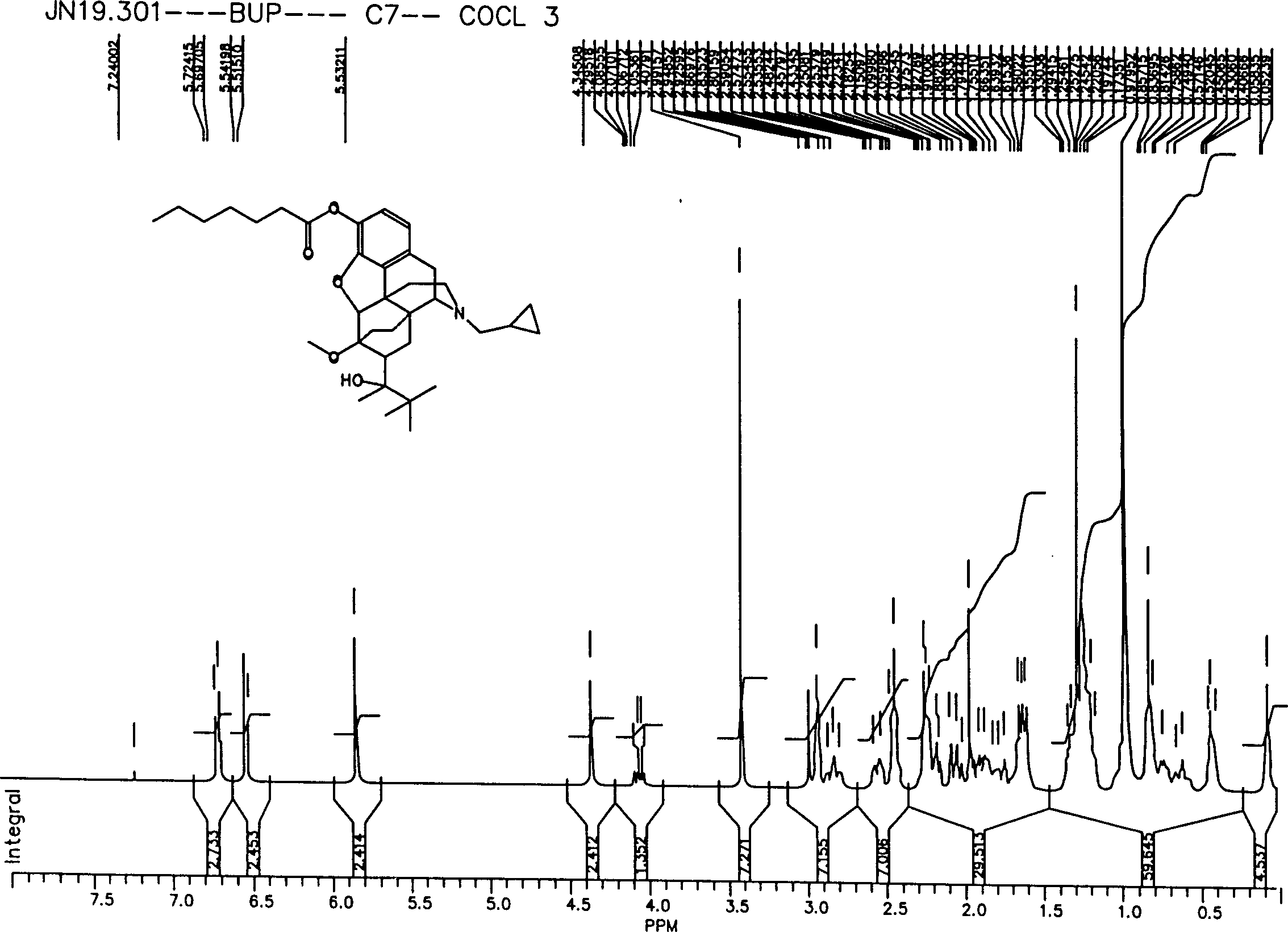
[0123] Synthesis Example 1: Preparation of Buprenorphine Enanthate
[0124] 75 mL of dichloromethane (Mallinckrodt, Baker, U.S.A.) and 0.01 M of buprenorphine HCl or base were added to a 250 mL round bottom flask placed in an ice bath for cooling. The mixture was stirred, and 20 mL of dichloromethane containing 0.03 M of triethylamine (Sigma, MO, U.S.A.) was added slowly. Another 20 mL of dichloromethane containing 0.011 moles of heptanoyl chloride (Aldrich, Milwaukee, U.S.A.) was added dropwise with rapid stirring. After that, the mixture was stirred at room temperature for 1 hour. 20 ml of a 10% sodium carbonate was then added to neutralize residual acid and remove water soluble impurities. Sodium sulfate was used to dehydrate the solution. After drying under vacuum, the title compound (ie buprenorphine enanthate) was obtained. The product was purified by column chromatography.
[0125] The title compound was generated by figure 1 , figure 2 , image 3 and Figure ...
Synthetic example 2
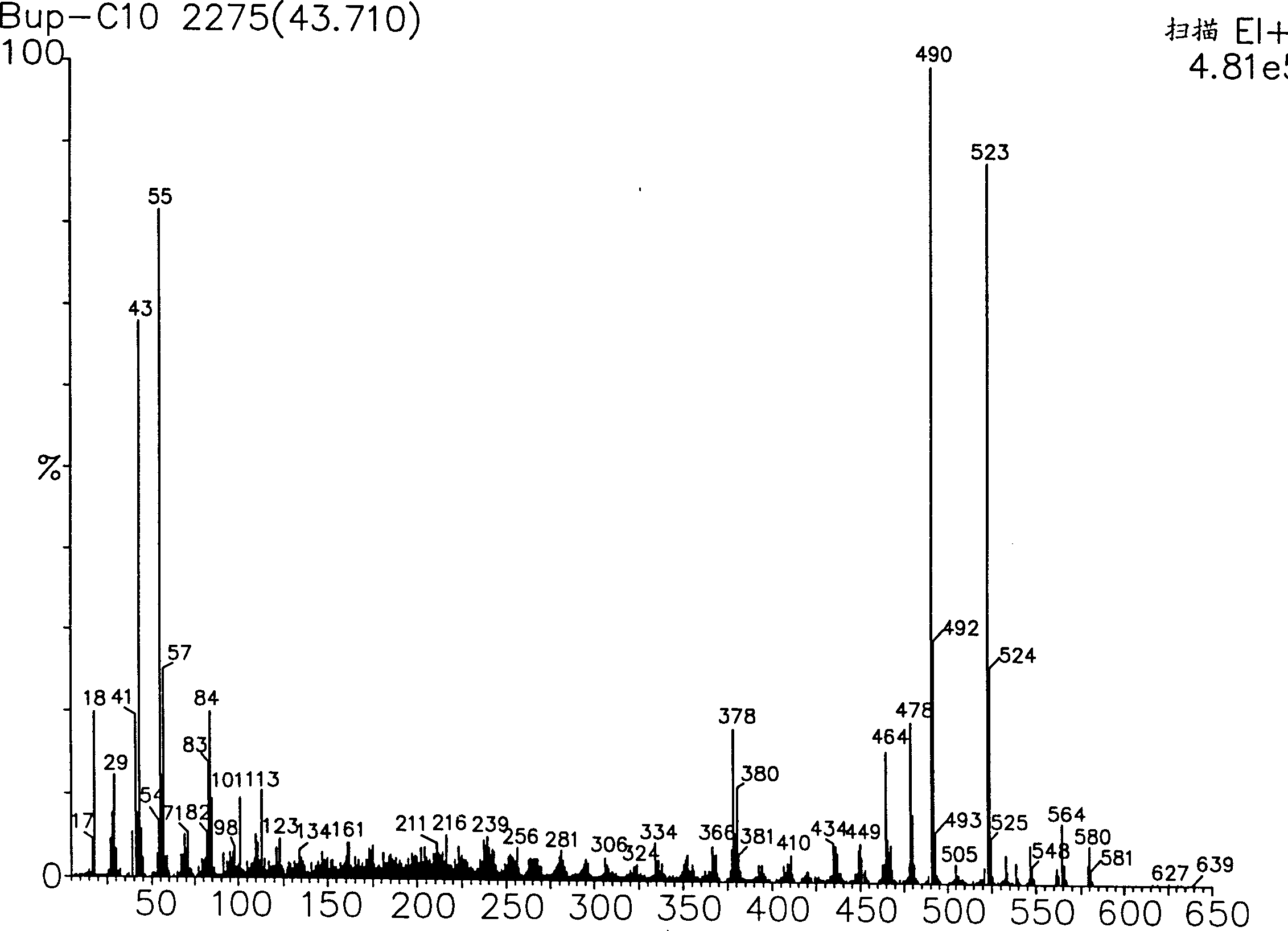
[0131] Synthesis Example 2: Preparation of Buprenorphine Decanoate
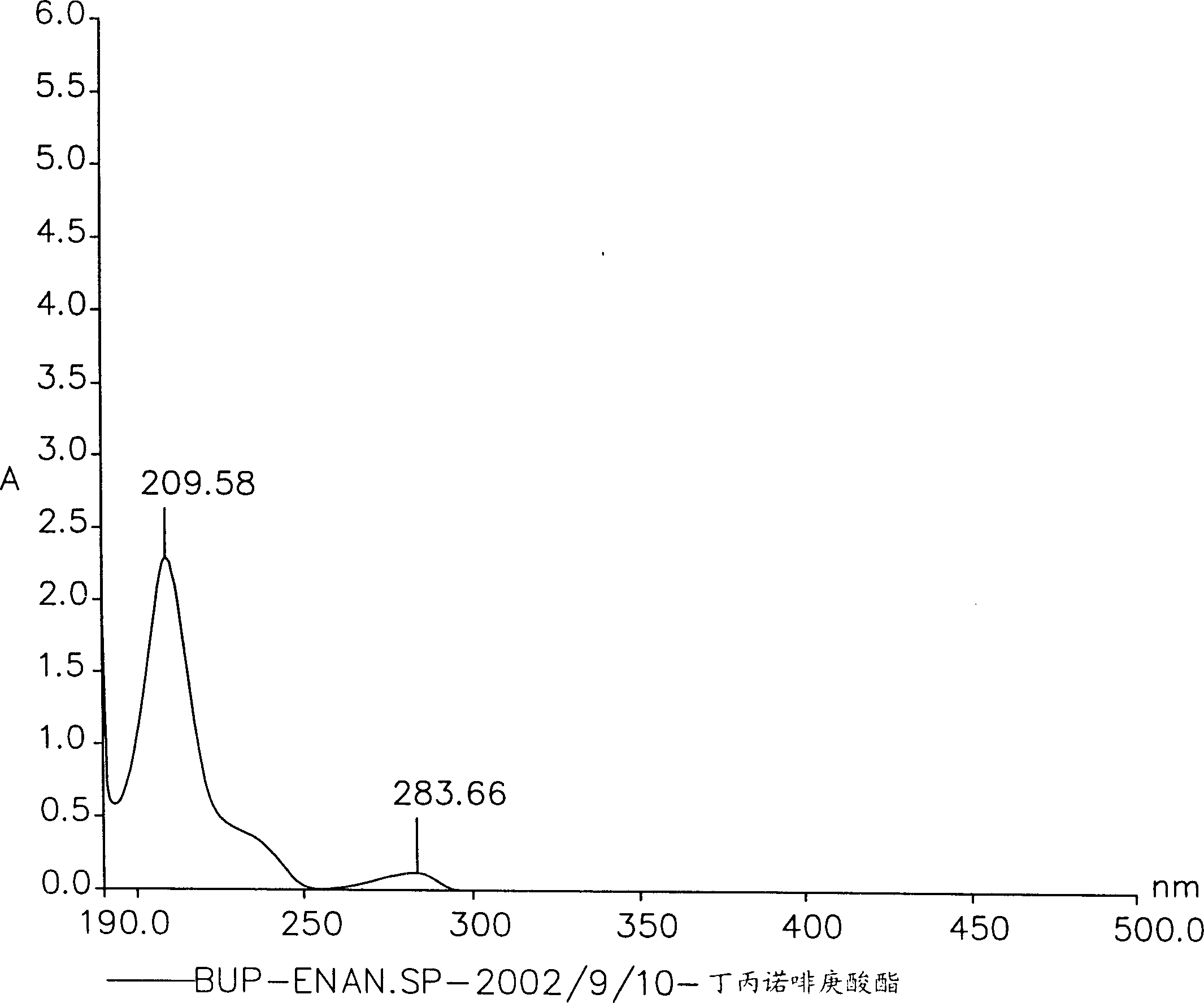
[0132] The title compound was prepared according to the method described in Synthesis Example 1 above, except that 0.011 mol of decanoyl chloride (Fluka, Buchs, Switzerland) was used instead of heptanoyl chloride. Pure buprenorphine decanoate was obtained (see Figure 5 , Image 6 , Figure 7 and Figure 8 , these figures respectively show the 1 H-NMR spectrum, mass spectrum, UV spectrum and IR spectrum).
[0133] Measured properties of the title compound:
[0134] Representative 1 H-NMR (400MHz, CDCl 3 ): 6.76(d, 1H, J=8.0Hz), 6.58(d, 1H, J=8.1Hz), 5.91(s, 1H), 4.41(s, 1H), 3.45(s, 3H), 3.00(m .2H), 2.87(m, 1H), 2.50(t, 2H, J=7.4Hz), 2.33-2.10(m.5H), 2.00-1.78(m, 4H), 1.72-1.66(m.4H), 1.37-1.25(m, 18H), 1.04(m, 11H), 0.87(t, 3H, J=6.6Hz), 0.80(m, 1H), 0.69(m, 1H), 0.48(m, 2H), 0.11 (m, 2H).
[0135]Representative mass spectral fragments (amu): 622, 607, 565, 533, 521, 507, 380, 55 [determination p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com