Mucosal disease treatment system and application thereof
A disease treatment and mucosal technology, applied in the field of medicine, can solve the problems of insignificant effect, high irritation, large first-pass effect of drugs, etc., to achieve a wide range of applications and the effect of promoting rehabilitation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] Application of mucosal disease treatment system in the treatment of oral ulcers
[0076] First, the preparation of bioadhesives loaded with biomedical materials:
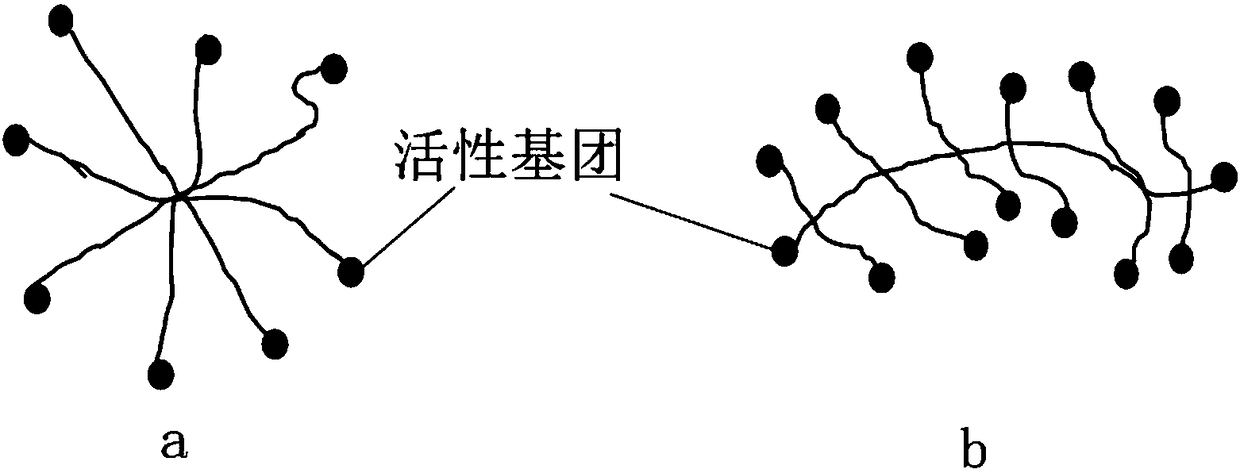
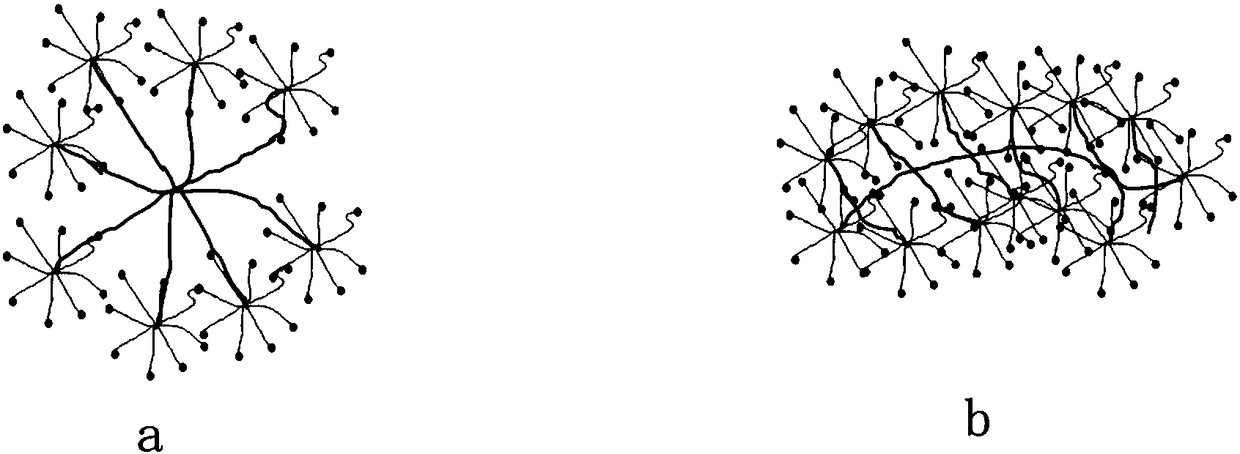
[0077] Component a: 2-20 parts by mass of sodium hyaluronate, 2-20 parts by mass of sodium alginate, and 20-50 parts by mass of hexadecylaminopolyamide-amine star polymer are dissolved in 1000 parts by mass of water;
[0078] Component b: 10-50 parts by mass of aldehyde-modified pullulan, 0.1-10 parts by mass of calcium pantothenate, 0.1-20 parts by mass of vitamin B2, and 0.1-20 parts by mass of vitamin C are dissolved in 1000 parts by mass of water.
[0079] Secondly, the method of use: cover the ulcer with a thin layer of component a through a dropper, cotton swab or spray bottle, and then spray component b through the spray bottle to quickly form a bioadhesive in situ on the ulcer , the adhesive firmly adheres to the ulcer, seals the ulcer, and releases sodium hyaluronate, sodium alginate, vitamin B2, an...
Embodiment 2
[0083] Application of mucosal disease treatment system in the treatment of oral injury mucosa
[0084] The oral mucosal injury in this embodiment mainly includes oral mucosal bites, scratches, punctures, bruises, etc., which have the characteristics of bleeding and rupture.
[0085] First, the preparation of bioadhesives loaded with biomedical materials:
[0086] Component a: take 20-50 parts by mass of hexadecylaminopolyamide-amine star polymer, 2-20 parts by mass of carboxymethyl chitosan, 2-20 parts by mass of sodium alginate, and 0.001-0.1 parts by mass of epidermal cells Growth factor (EGF), 0.001-0.1 parts by mass of fibroblast growth factor (bFGF), dissolved in 1000 parts by mass of water;
[0087] Component b: 10-50 parts by mass of aldehyde-modified pullulan and 0.1-10 parts by mass of calcium pantothenate are dissolved in 1000 parts by mass of water.
[0088] Secondly, how to use: cover the wound with a thin layer of component a through a dropper, cotton swab or sp...
Embodiment 3
[0091] Application of mucosal disease treatment system in the treatment of pharyngitis
[0092] First, the preparation of bioadhesives loaded with biomedical materials:
[0093] Component a: Take 10-100 parts by mass of ribavirin, 5-50 parts by mass of mercaptolated pullulan, 5-50 parts by mass of mercaptolated dextran, and 0.5-5 parts of sodium hyaluronate, dissolve in 1000 parts by mass of water;
[0094] Component b: 20-100 parts by mass of vinylsulfonated pullulan and 0.1-10 parts by mass of sodium alginate are dissolved in 1000 parts by mass of water.
[0095] Secondly, how to use: Spray component a to the throat through a spray bottle with a slender nozzle, and then spray component b to the throat to quickly form a bioadhesive in situ on the throat, and the adhesive is stable Adheres to the inflamed throat, protects the throat, and releases ribavirin and other medical materials.
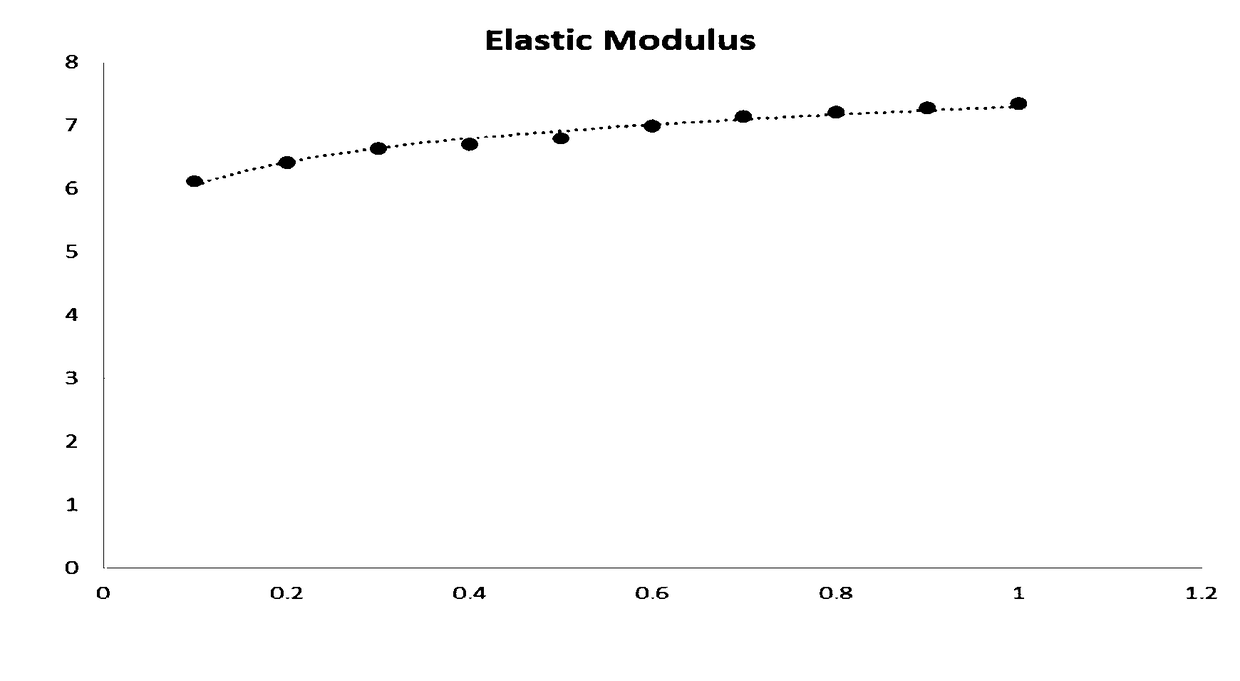
[0096] The mechanical properties of the bioadhesive loaded with biomedical materials for...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com