Patents
Literature
385 results about "Glucosidases" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Glucosidases are the glycoside hydrolase enzymes categorized under the EC number 3.2.1.
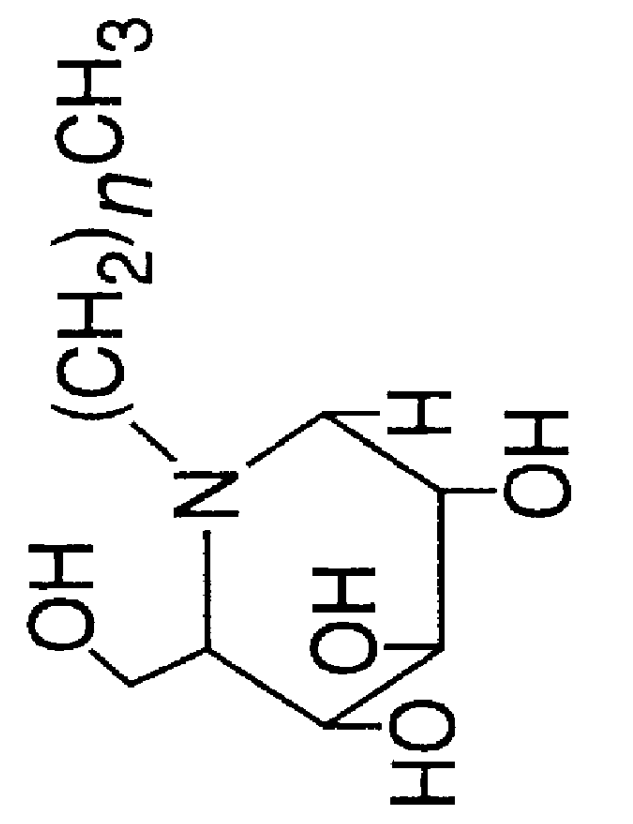
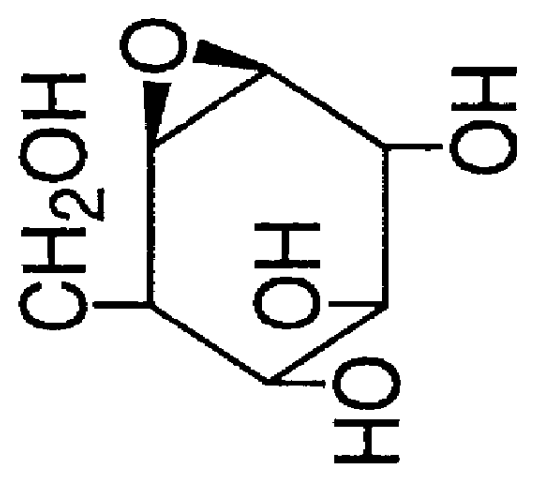
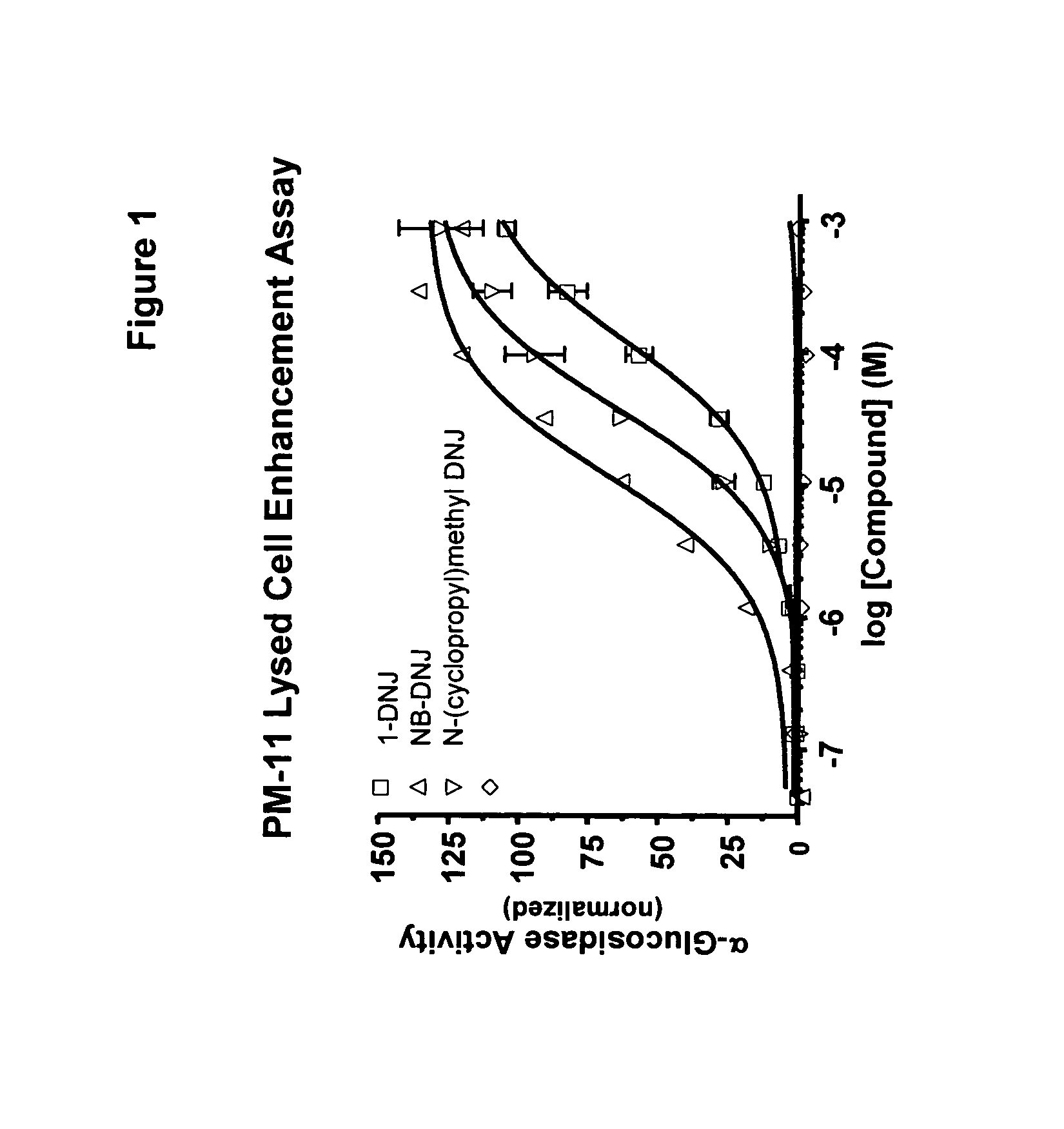
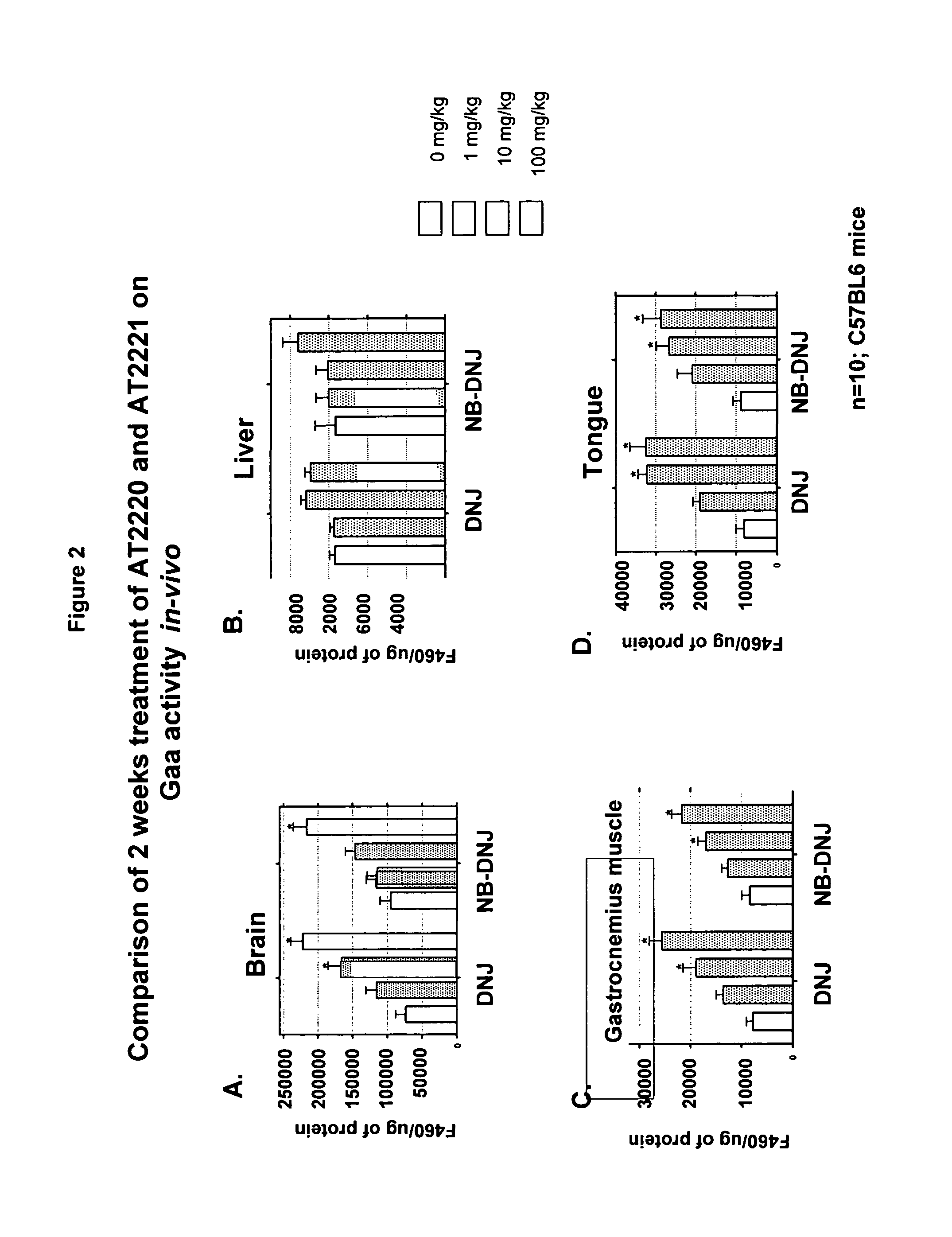
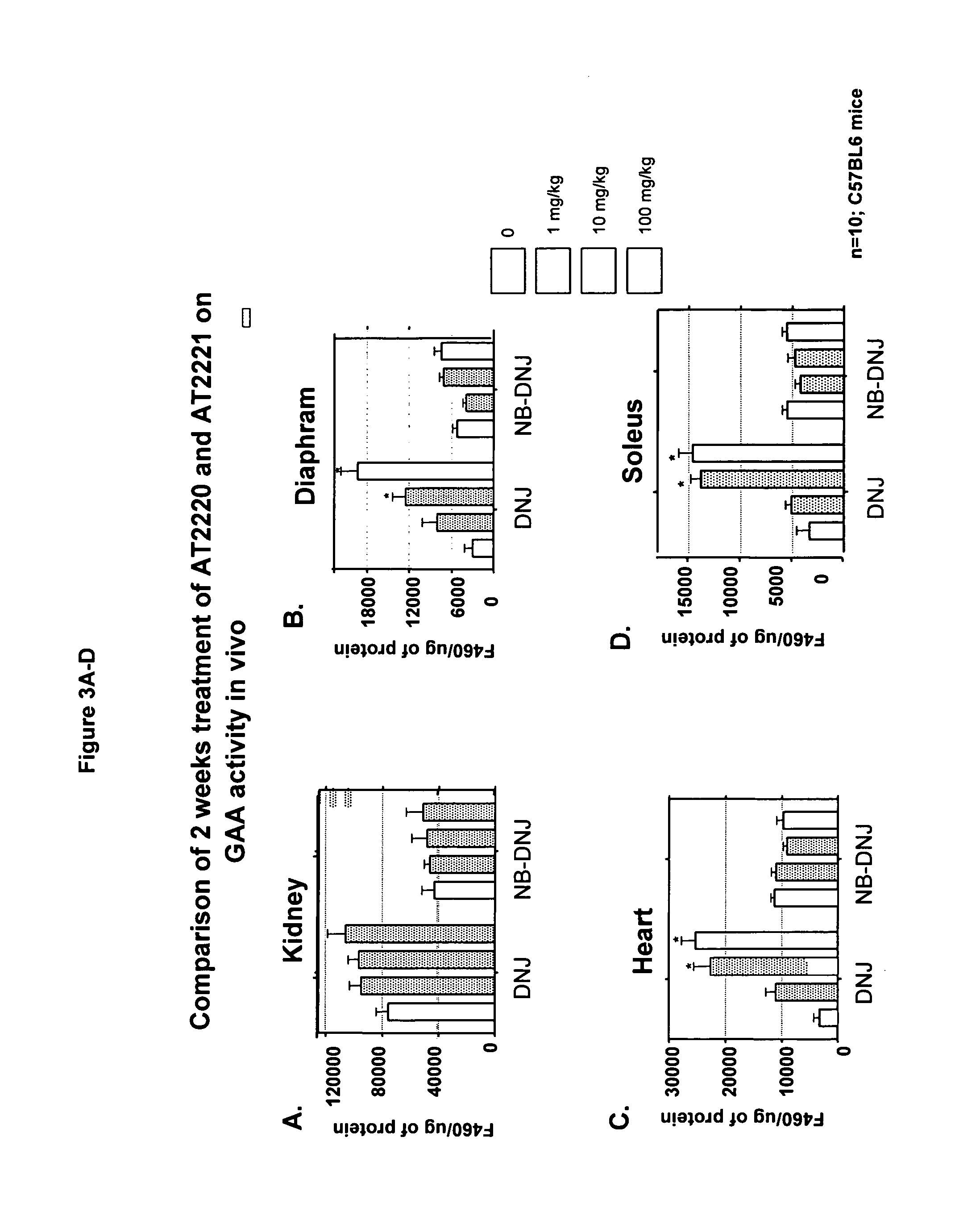
Method for the treatment of Pompe disease using 1-deoxynojirimycin and derivatives
ActiveUS20060264467A1Increased GAA activityRelieve symptomsBiocideNervous disorderDeoxynojirimycineWild type
The present invention provides a method for increasing the activity of a mutant or wild-type α-glucosidase enzyme in vitro and in vivo by contacting the enzyme with a specific pharmacological chaperone which is a derivative of 1-deoxynojirimycin. The invention also provides a method for the treatment of Pompe disease by administration of chaperone small molecule compound which is a derivative of 1-deoxynojirimycin. The 1-deoxynojirimycin derivative is substituted at the N or C1 position. Combination therapy with replacement α-glucosidase gene or enzyme is also provided.
Owner:AMICUS THERAPEUTICS INC
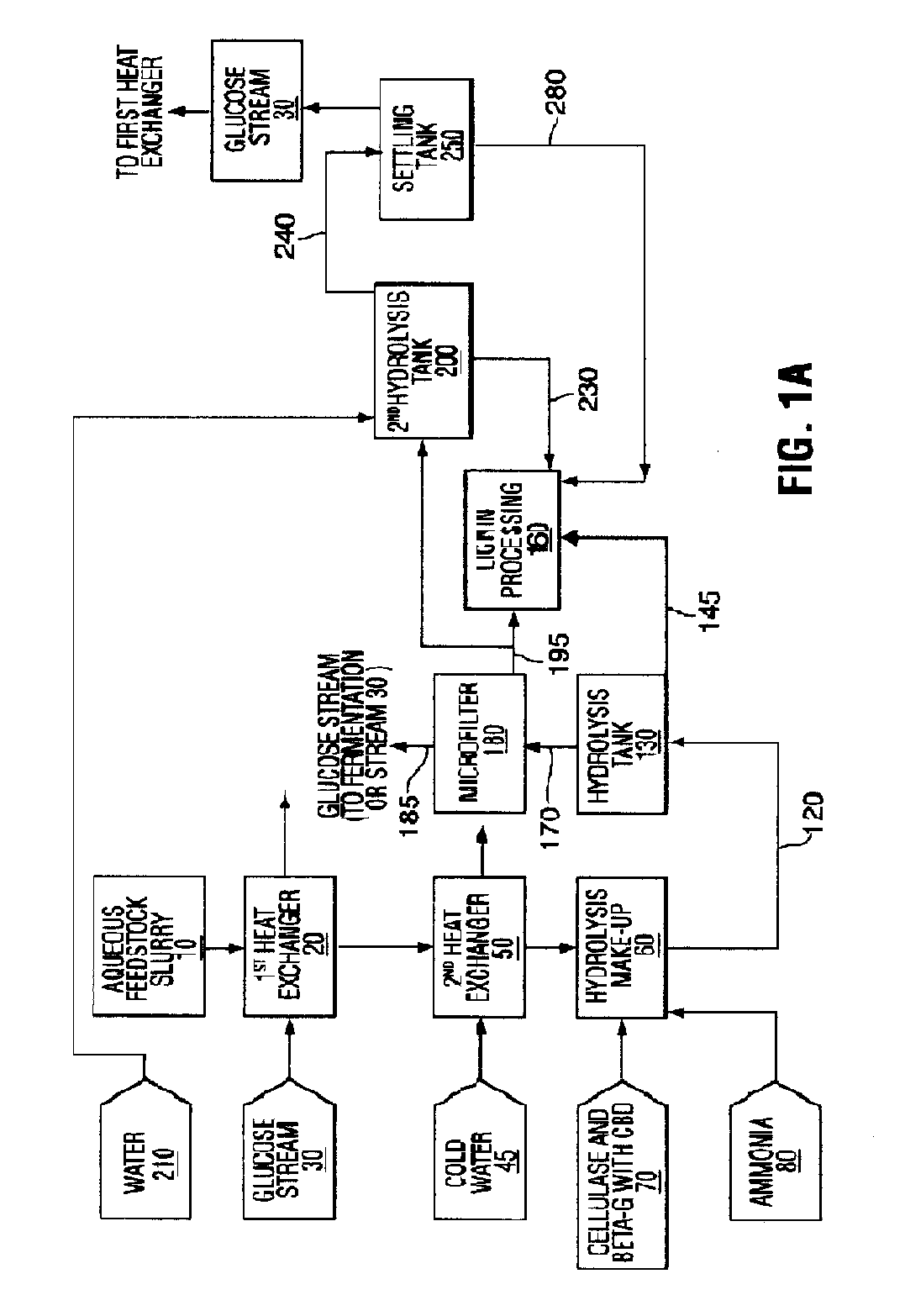
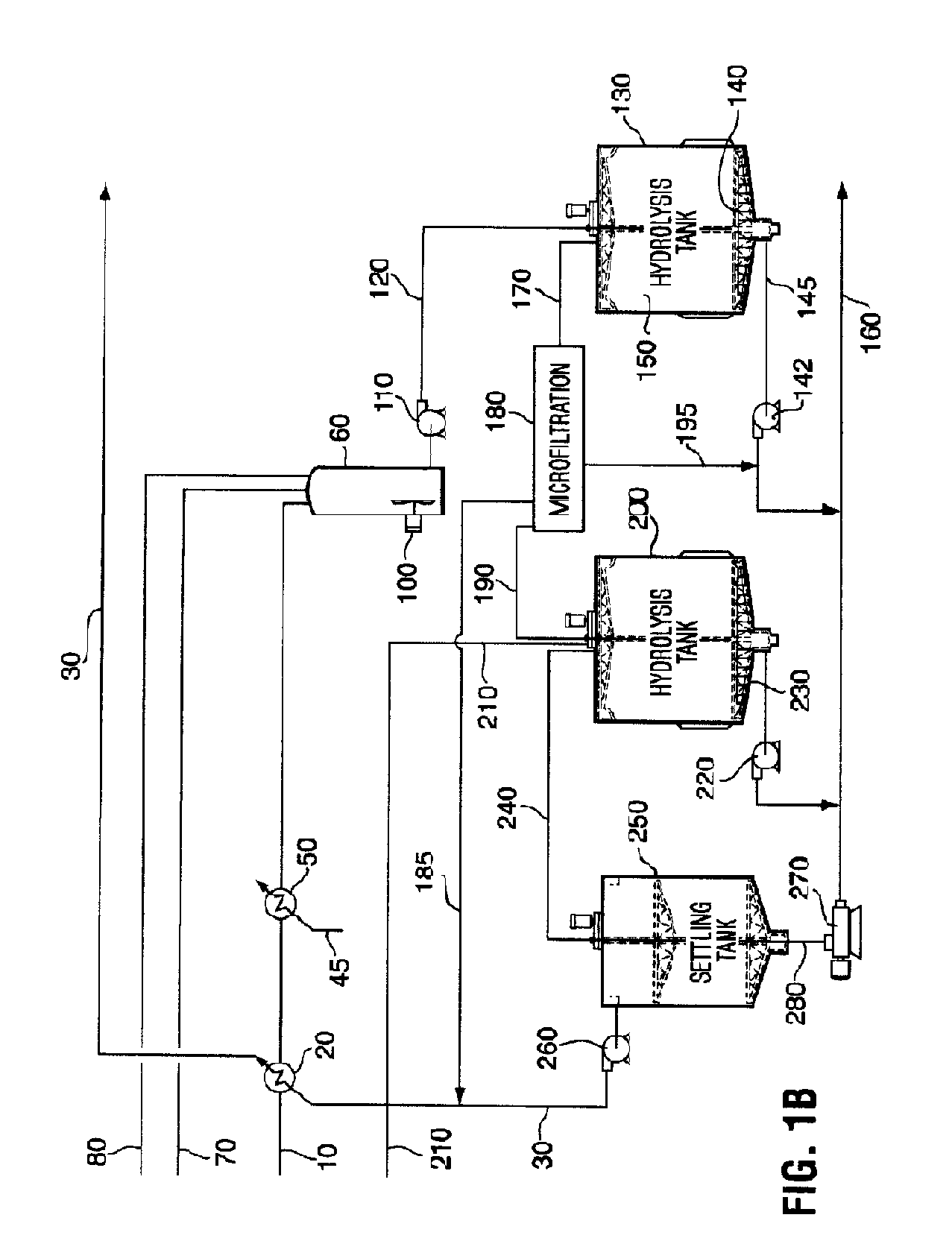
Enzyme compositions for the improved enzymatic hydrolysis of cellulose and methods of using same
InactiveUS20090209009A1Extended reaction timeReduce probabilityBiofuelsChemical recyclingFiberEnzymatic hydrolysis
A process for the enzymatic hydrolysis of cellulose to produce a hydrolysis product comprising glucose from a pretreated lignocellulosic feedstock and enzymes for use in the process are provided. The process comprises hydrolyzing an aqueous slurry of a pretreated lignocellulosic feedstock with cellulase enzymes, one or more than one β-glucosidase enzyme and a binding agent for binding the β-glucosidase enzyme to fiber solids present in the aqueous slurry. During the hydrolysis, both the cellulase enzyme and β-glucosidase enzyme bind to the fiber solids. The hydrolysis is performed in a solids-retaining hydrolysis reactor so that unhydrolyzed fiber solids and bound enzyme are retained in the reactor longer than the aqueous phase of the slurry.
Owner:IOGEN ENERGY CORP
Concomitant drugs
InactiveUS20050197376A1Good treatment effectReduce the amount requiredBiocideMetabolism disorderConcomitant drugGlycosidase inhibitor
This invention provides a pharmaceutical agent containing, in combination, a sulfonamide compound and other therapeutic agent, preferably, at least one compound represented by the formula (I): R1—SO2—NH—CO-A1-CH2—R2 [each symbol is as defined in the specification] or a pharmaceutically acceptable salt thereof, and at least one pharmaceutical agent selected from the group consisting of an α-glucosidase inhibitor, an insulin secretagogue, a sulfonylurea and a biguanide, which has a superior therapeutic effect.
Owner:ASTELLAS PHARMA INC
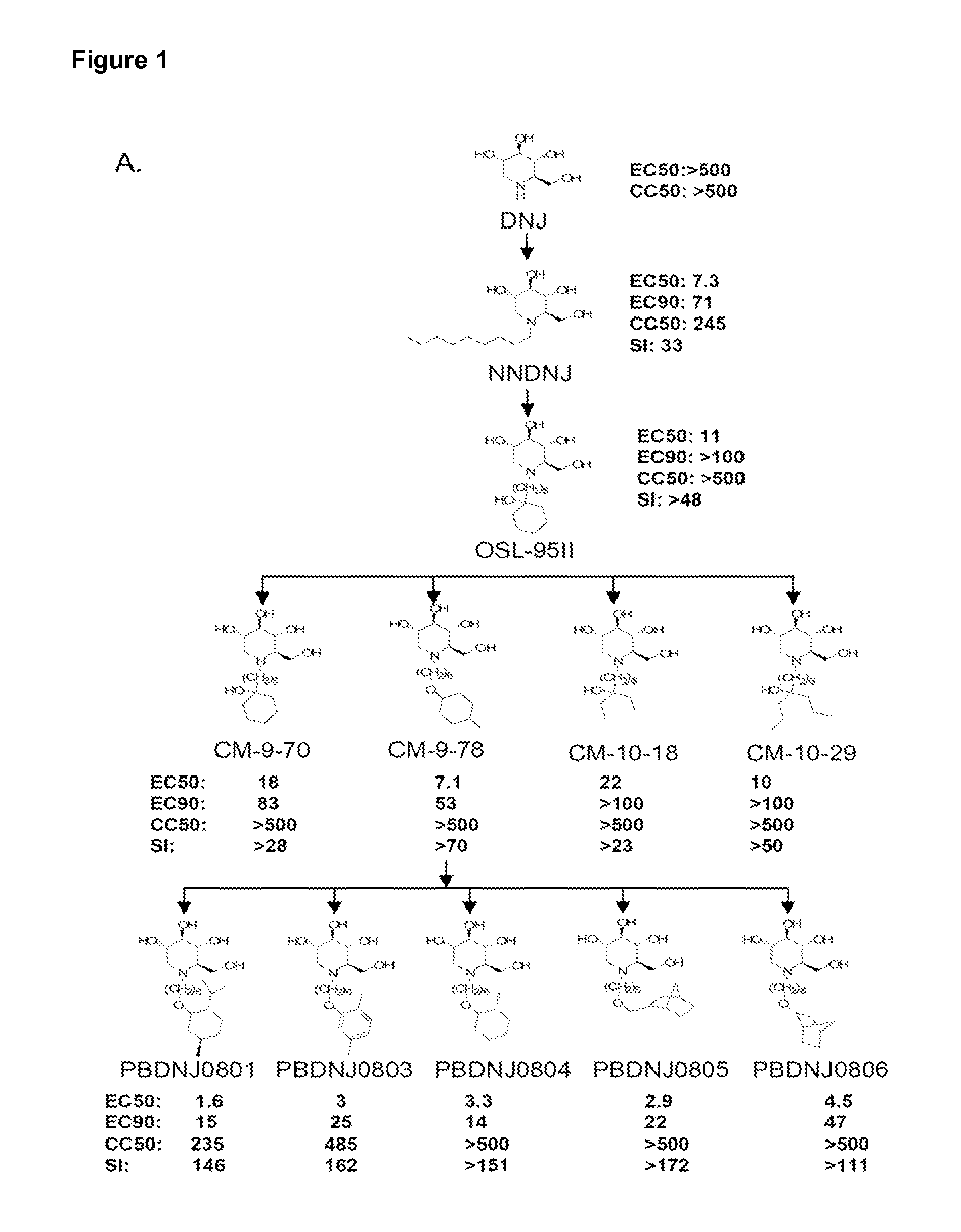
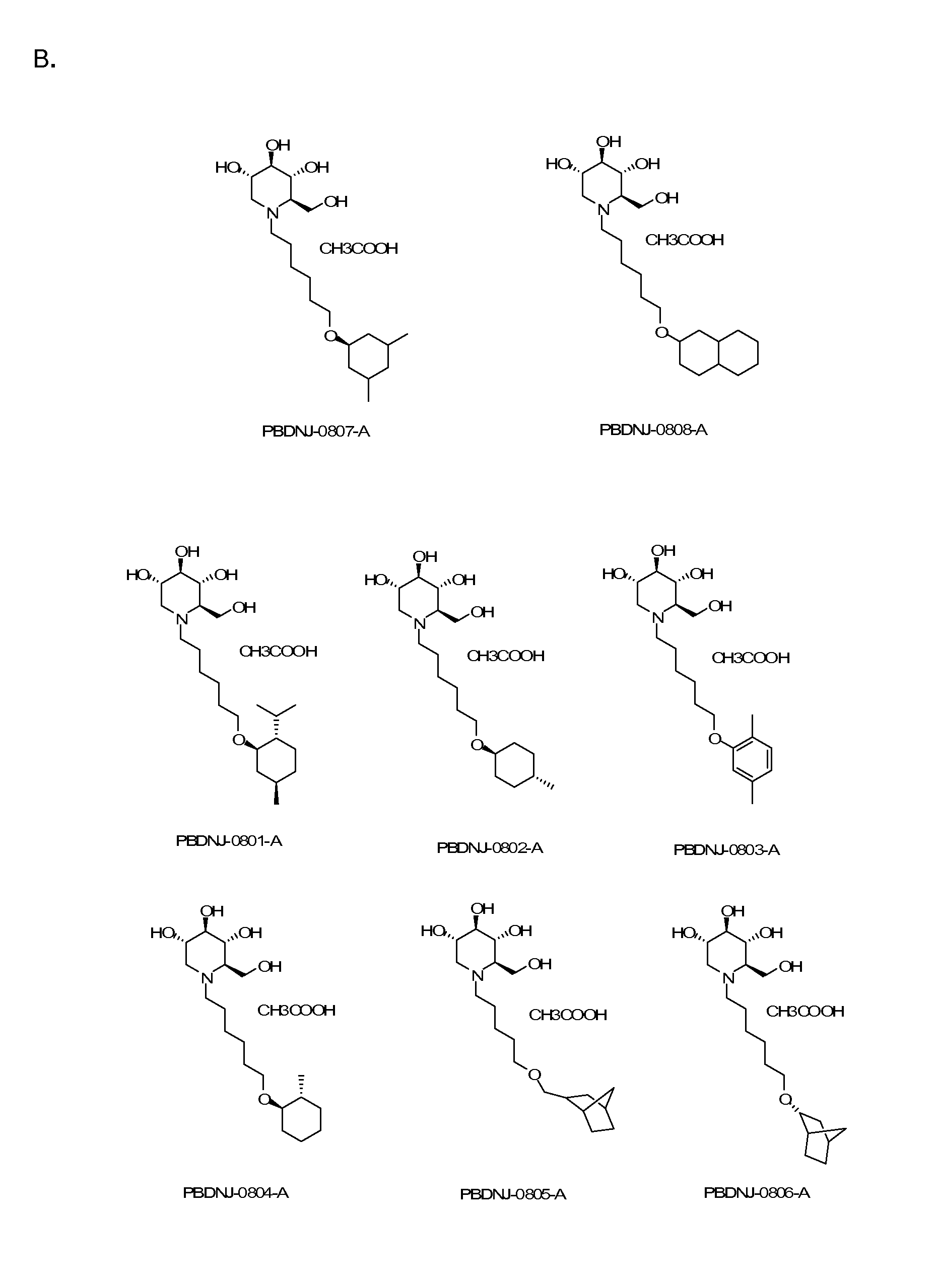
Novel Imino Sugar Derivatives Demonstrate Potent Antiviral Activity and Reducted Toxicity
InactiveUS20110189771A1Good effectImprove performanceOrganic chemistryTissue cultureBovine Viral Diarrhea VirusesSide chain
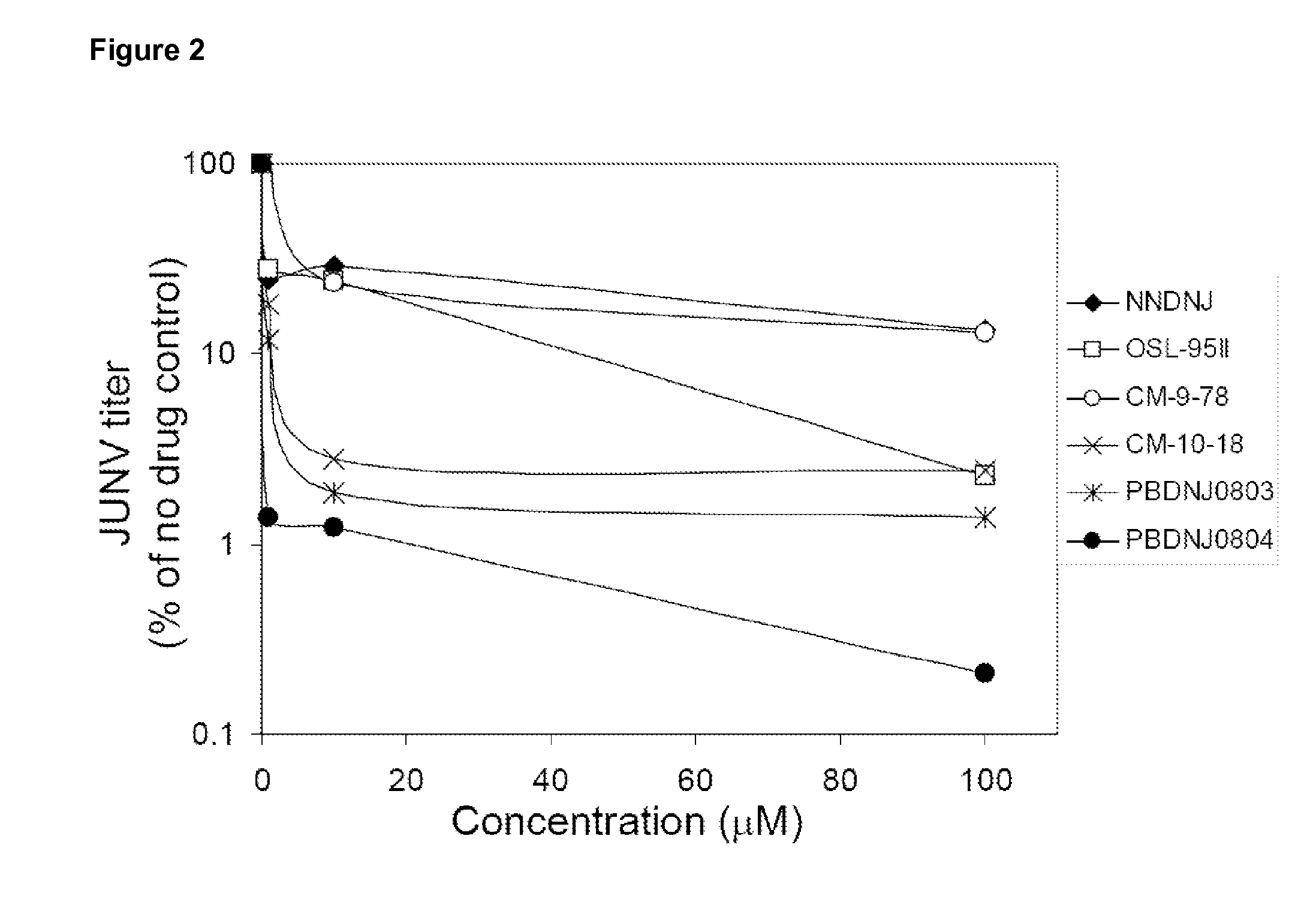
Imino sugars, such as deoxynojirimycin (DNJ), are glucose analogues that selectively inhibit cellular α-glucosidase I and II (enzymes that process N-linked glycans in glycoprotein) and exhibit broad spectrum antiviral activities against many enveloped viruses. Previously we have reported a novel DNJ derivative, OSL-95II, with antiviral activity and reduced cytotoxicity. In order to develop imino sugars with more potent antiviral activity as well as improved toxicity profile, OSL-95II was modified by diversifying the nitrogen linked alkylated side chain. The antiviral activities were initially tested in bovine viral diarrhea virus (BVDV) infected MDBK cells, yielding several imino sugar derivatives with novel structure and superior antiviral activity and toxicity profile. Furthermore, these new compounds were shown to be active against Dengue virus (DV) and West Nile virus (WNV) infection in BHK cells where potent anti-DV activity having submicromolar EC50 values and SI of greater than 900. These compounds represent a new generation of iminio sugars and their analogues, having application in the clinical treatment of infection of DV and other members of flaviviridae.
Owner:INST FOR HEPATITS & VIRUS RES +1
Polypeptides having beta-glucosidase activity and polynucleotides encoding same
The present invention relates to isolated polypeptides having beta-glucosidase activity and isolated polynucleotides encoding the polypeptides. The invention also relates to nucleic acid constructs, vectors, and host cells comprising the polynucleotides as well as methods for producing and using the polypeptides.
Owner:NOVO NORDISKBIOTECH INC
Total flavone extract of maniod eibish, its preparation and application
InactiveCN1994337ASignificant effect on the treatment of nephritisClear certaintySugar derivativesPill deliveryMedicineGlycoside formation
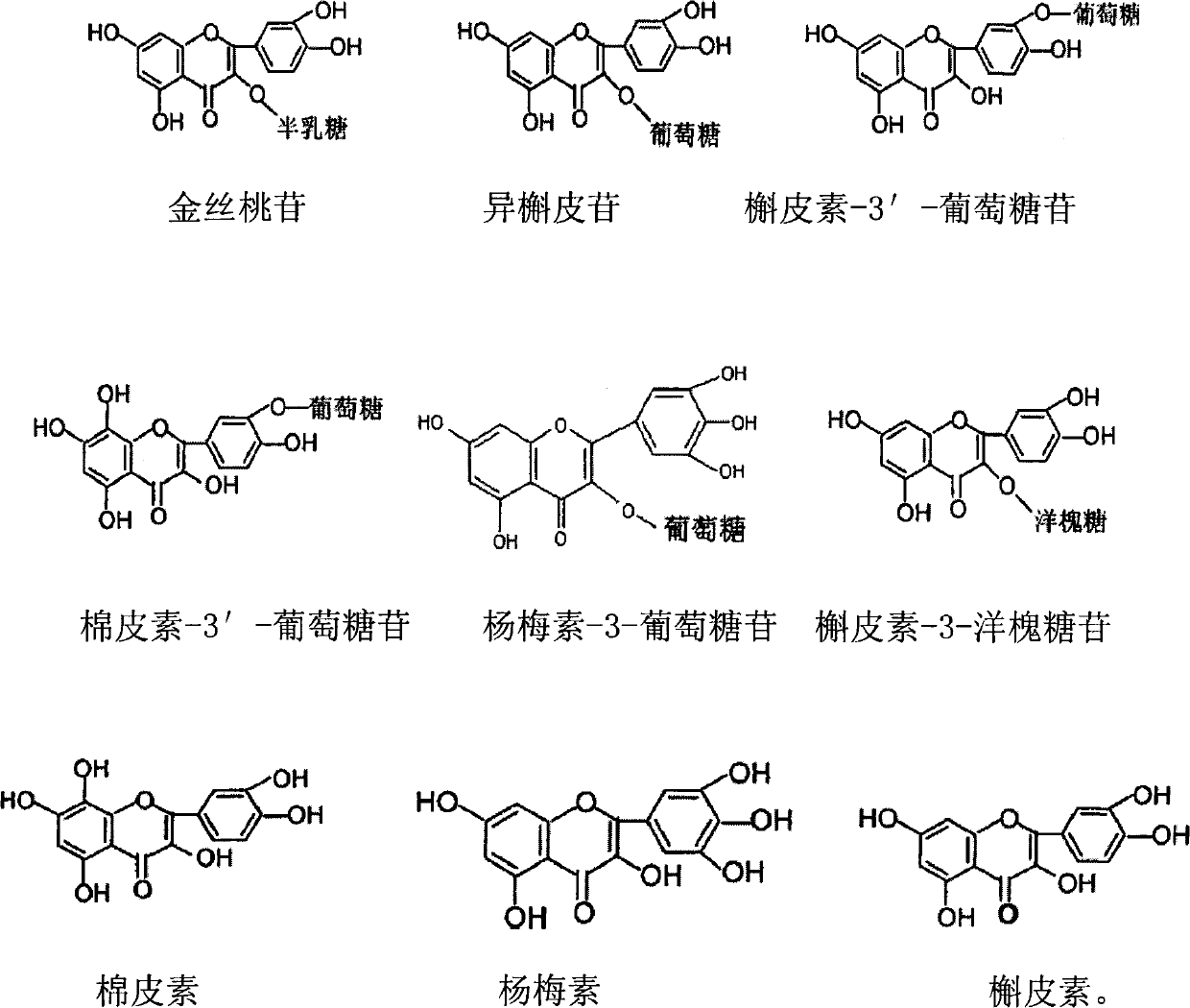
The invention relates to a hollyhock sunflower chromocor extractive, wherein it is characterized in that: the chromocor content is 50-90%; the chromocor comprises 1. 0-5. 0% meletin-3-acacia glycoside, 8-24. 0% hyperin, 7. 0-20.0% isoquercitrin, 5. 0-15. 0% meletin-3-glucosidase, 3. 0-10.0% cotton-3-glucosidase, 0.5-5. 0% gale element, 0.5-5. 0% cotton, and 2. 0-8. 0% meletin, etc. The inventive extractive can be used to prepare the drug that treats nephritis, with high reliability.
Owner:周亚球 +1
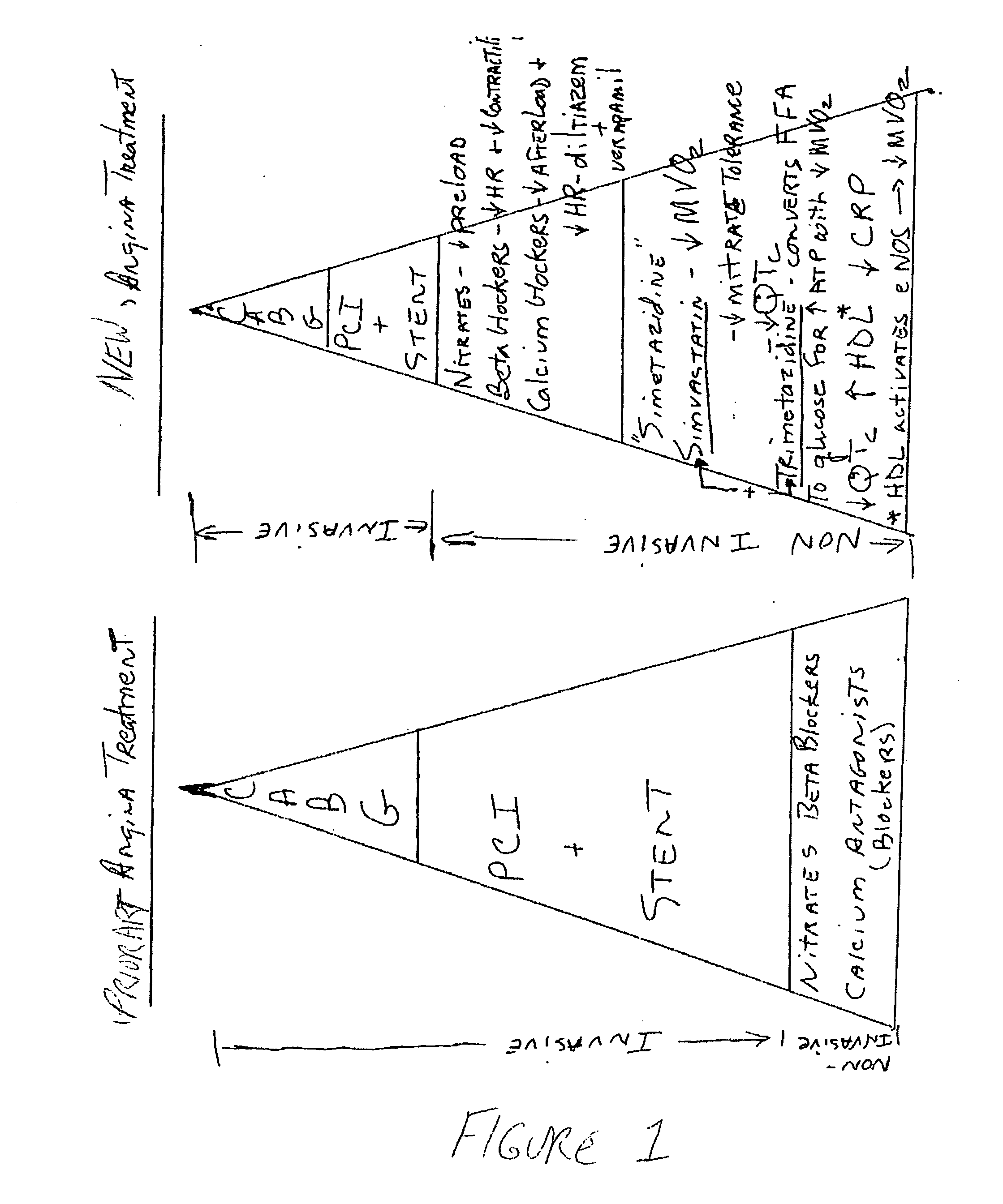
Combination therapy for endothelial dysfunction, angina and diabetes
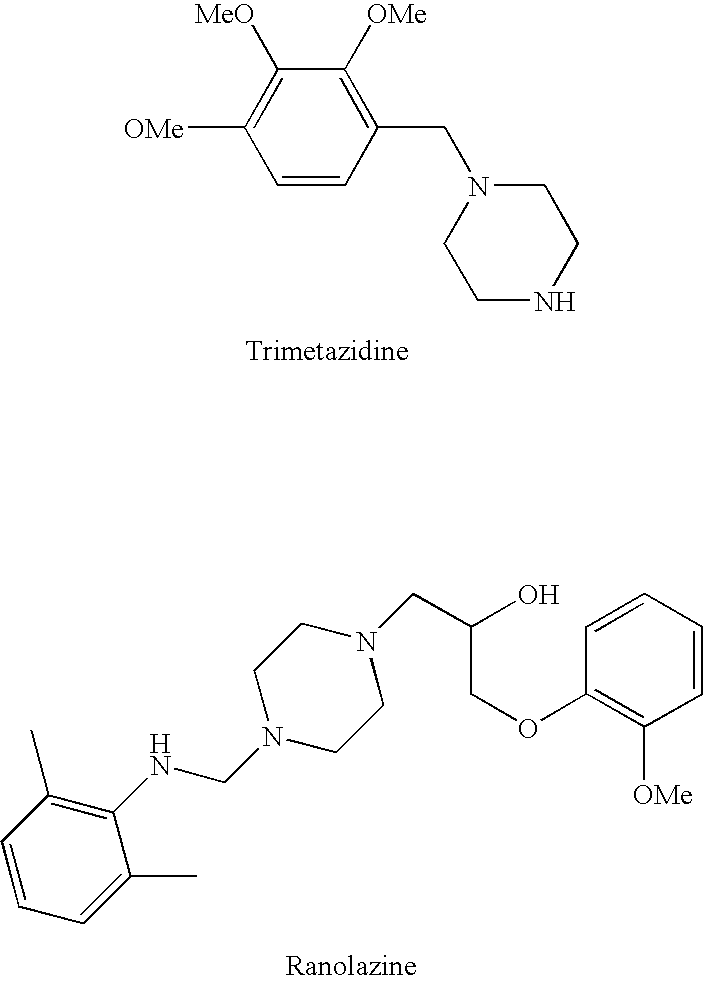
InactiveUS20060205727A1Control blood sugar levelsIncrease productionBiocideMetabolism disorderHMG-CoA reductaseTrimetazidine
The combination of a HMG CoA reductase inhibitor like a statin, such as simvastatin, with a pFox inhibitor such as trimetazidine (“Simetazidine”) is particularly advantageous for treatment of end-stage complications, such as acute coronary syndrome (ACS) and chronic angina, especially in type II diabetics. The combination therapy is also useful in the treatment and / or prevention of chronic heart failure (CHF) and peripheral arterial disease (PAD). The combination of a nitric oxide (NO) mechanism with increased NO production with pFox inhibition simultaneously treats both the effect and the cause of angina. One or more oral hypoglycemic compounds (biguanides, insulin sensitizers, such as thiazolidinediones, α-glucosidase inhibitors, insulin secretagogues, and dipeptidyl peptidase IV inhibitors), protein kinase C (PKC) inhibitors, and acetyl-CoA carboxylase inhibitors can also be used in combination with the HMG CoA reductase inhibitors and / or pFox inhibitors, especially in type II diabetics, to control glucose levels and treat endothelial dysfunction. The drugs can be given in combination (e.g. a single tablet) or in separate dosage forms, administered simultaneously or sequentially. In the preferred form the statin is given in a dose of between 5 and 80 mg / day in two separate doses, and the pFox inhibitor is administered in a sustained or extended dosage formulation at a dose of 20 mg three times a day or 35 mg two times a day. The dose of the oral hypoglycemic, PKC inhibitor, or acetyl-CoA carboxylase inhibitor varies with the type of drug used.
Owner:HONG KONG NITRIC OXIDE
Method for producing ethanol from lignocellulose biomaterial by use of neu-heat-resistant enzyme
It includes the stages of grinding the lignocellulosic biomass to a size of 15-30 mm, subjecting the product obtained to steam explosion pre-treatment at a temperature of 190-230 DEG C for between 1 and 10 minutes in a reactor (2), collecting the pre-treated material in a cyclone (3) and separating the liquid and solid fractions by filtration in a filter press (9), introducing the solid fraction in a fermentation deposit (10), adding a cellulase at a concentration of 15 UFP per gram of cellulose and 12.6 International Units of beta -glucosidase enzyme dissolved in citrate buffer pH 4.8, inoculating the fermentation deposit (10) with a culture of the heat-tolerant bacteria Kluyveromyces marxianus CECT 10875, obtained by chemical mutagenesis from strain DER-26 of Kluyveromyces marxianus and shaking the mixture for 72 hours at 42 DEG C.
Owner:RES CENT OF ENERGY SOURCE ENVIRONMENT & TECH
Methods of use and nutritional compositions of touchi extract
InactiveUS20090148545A1Reduce complicationsReduce post-prandial glucose excursionBiocideAntiviralsPhysiologyConstipation
Disclosed is a method and composition for nutritional compositions containing -glucosidase inhibitors, and more specifically Touchi Extract and its uses in the treatment of many disorders. These disorders include diabetes, hyperlipidemia, obesity, Metabolic syndrome / Syndrome X, COPD, malabsorption, Crohn's disease, diarrhea, constipation, irritable bowel syndrome, human immunodeficiency virus, cystic fibrosis, non-alcoholic steatohepatitis, polycystic ovarian syndrome including associate infertility, and erectile dysfunction. Further, -glucosidase inhibitors, and more specifically Touchi Extract can be used to aid healing in critical care patients and for general wound healing. Additionally, -glucosidase inhibitors, including Touchi Extract can be used to enhance athletic performance.
Owner:NESTEC SA
Methods and compositions for stimulating tissue growth and epithelial moisturization
InactiveUS6054433AImproved skin thicknessImprove flatnessBiocideHydroxy compound active ingredientsCosmetic appearancePhoto ageing
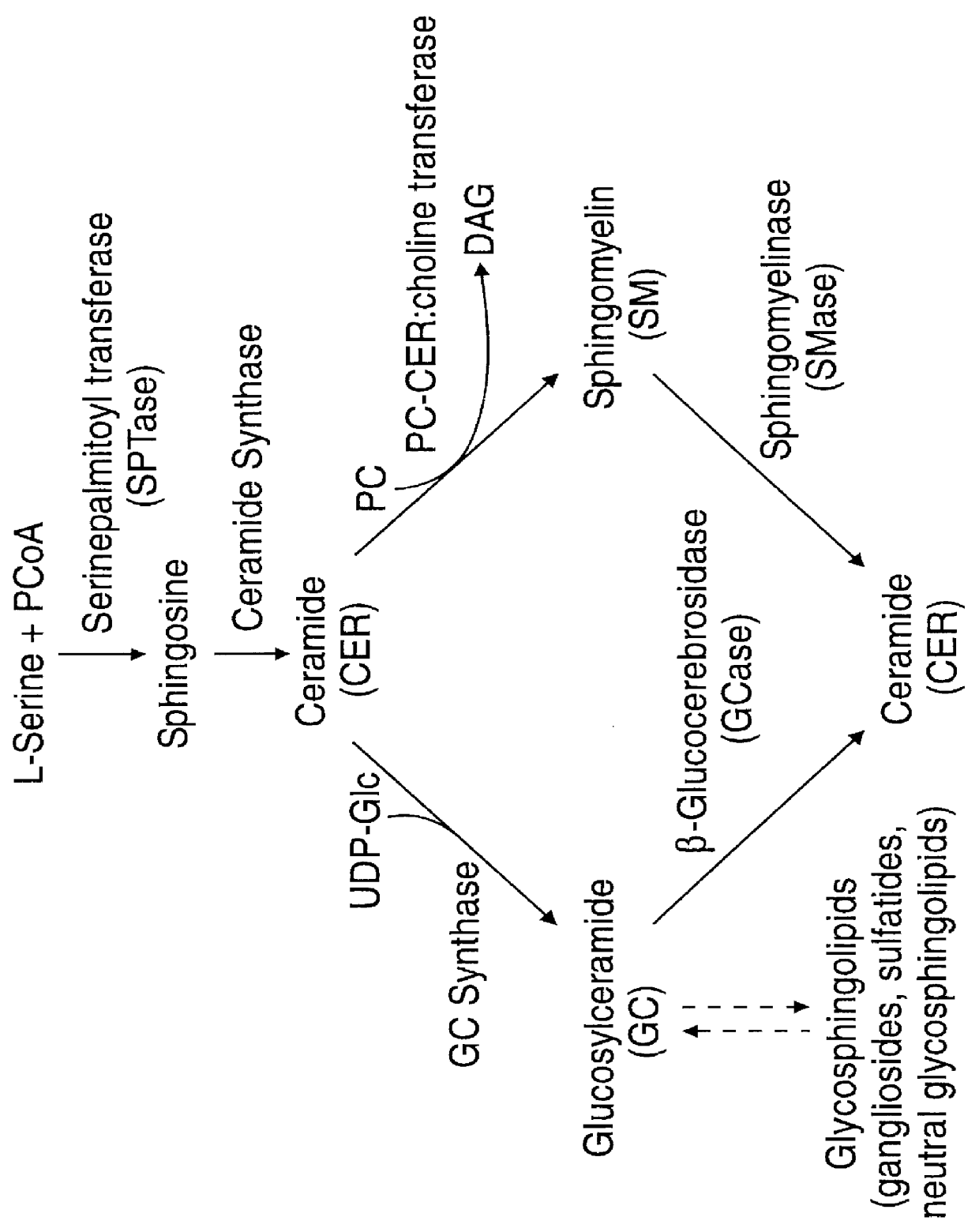
The invention herein encompasses methods effective to stimulate epithelial cell proliferation and / or enhance epithelial moisturization and lubrication in a mammalian subject utilizing a composition comprising one or more inhibitors of beta -glucosidase activity or beta -glucocerebrosidase activity. The composition of the method may alternatively comprise a glycosphingolipid, particularly glucocerebroside, or a combination of the above inhibitor(s) and a glycosphingolipid. The method is effective to enhance the cosmetic appearance of skin and promote healing of skin and mucous membranes damaged or deficient from aging, traumatic wounds, photo-aging and a variety of atrophic conditions. The method may be applied to cells in culture. Also included in the invention is a composition comprising one or more inhibitors of beta -glucosidase and a glycosphingolipid useful to stimulate cell proliferation and enhance tissue moisturization and lubrication.
Owner:RGT UNIV OF CALIFORNIA
Combination therapeutic compositions and method of use
The present invention provides pharmaceutical compositions and methods for the treatment of diabetes mellitus using combination therapy. The compositions relate to a compound of Formula I selected from one or more of betaines, lipidic betaines, betaine lipids and an antidiabetic agent such as sulfonylureas, biguanides, glitazones, .alpha.-glucosidase inhibitors, potassium channel antagonists, aldose reductase inhibitors, glucagon antagonists, activators of RXR, insulin therapy or other anti-obesity agent. The methods include the administration of the combination of compound of Formula I with antidiabetic agent where the two components are delivered in a simultaneous manner, where the compound of Formula I is administered first, followed by the antidiabetic agent, as well as wherein the antidiabetic agent is delivered first followed by the compound of Formula I.
Owner:MESSADEK JALLAL
Method for the treatment of pompe disease using 1-deoxynojirimycin and derivatives
The present invention provides a method for increasing the activity of a mutant or wild-type α-glucosidase enzyme in vitro and in vivo by contacting the enzyme with a specific pharmacological chaperone which is a derivative of 1-deoxynojirimycin. The invention also provides a method for the treatment of Pompe disease by administration of chaperone small molecule compound which is a derivative of 1-deoxynojirimycin. The 1-deoxynojirimycin derivative is substituted at the N or C1 position. Combination therapy with replacement α-glucosidase gene or enzyme is also provided.
Owner:AMICUS THERAPEUTICS INC
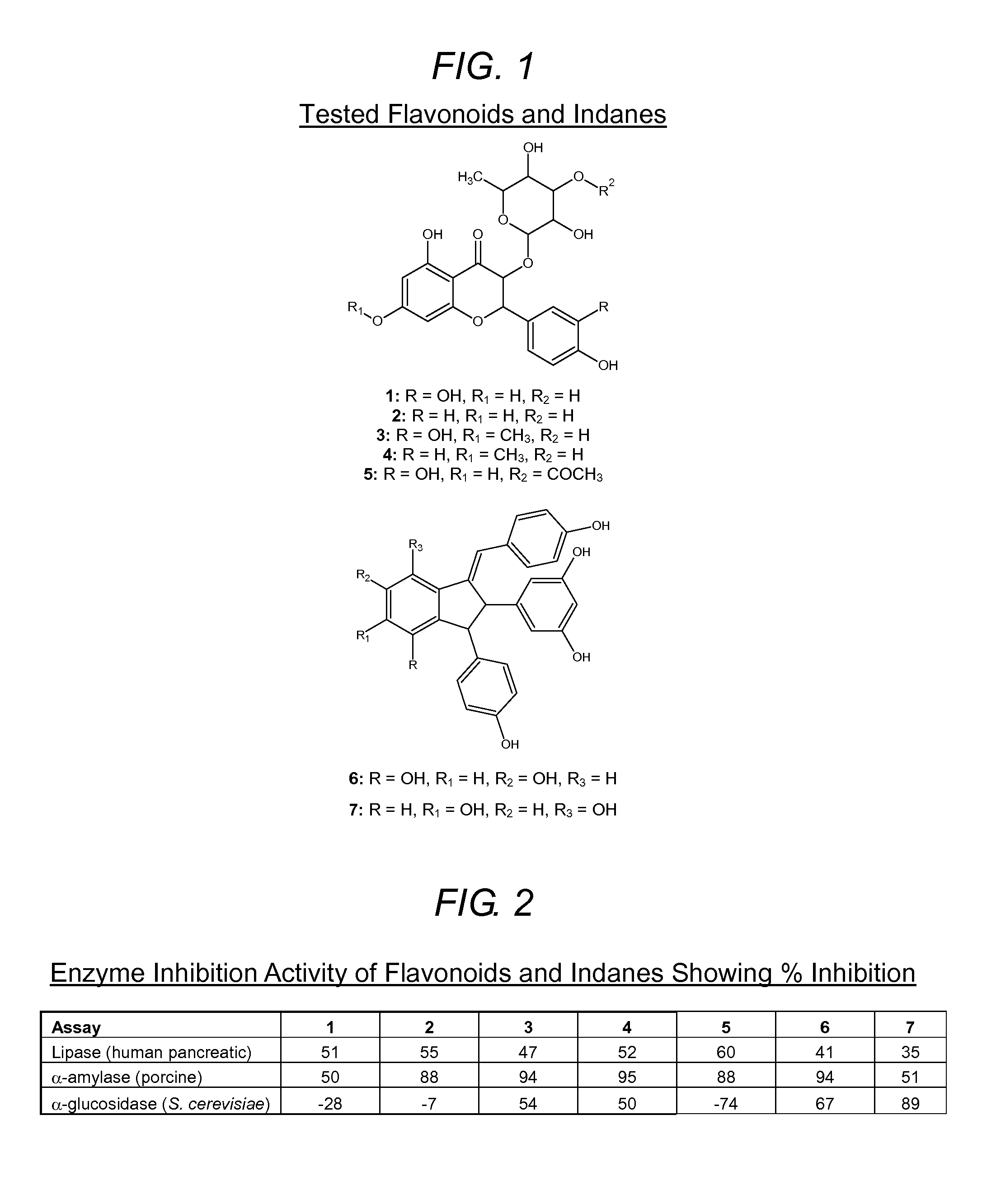
Methods and related compositions using specific flavonoids and indanes to reduce weight and inhibit lipase, alpha-amylase and alpha-glucosidase activity in mammals
The present invention relates generally to methods and related compositions using flavonoids and / or indanes extracted from the stems and leaves of C. quadrangularis to reduce weight and inhibit lipase, α-amylase and α-glucosidase activity in mammals. By example and not by way of limitation, embodiments of the present disclosure, a composition and related methods for reducing body weight and / or inhibiting any combination of lipase, α-amylase and α-glucosidase is provided. The composition contains an effective amount of one or more flavonoids or indanes selected from 3-O-rhamnopyranosylkaempferol, 3-(4-hydroxybenzylidene)-2-(2,5-dihydroxyphenyl)-1-(4-hydroxyphenyl)indane-4,6-diol, quercitrin, rhamnitrin, rhamnocitrin, quercitrin-3-O″-acetate and parthenocissin A.
Owner:GATEWAY HEALTH ALLIANCES
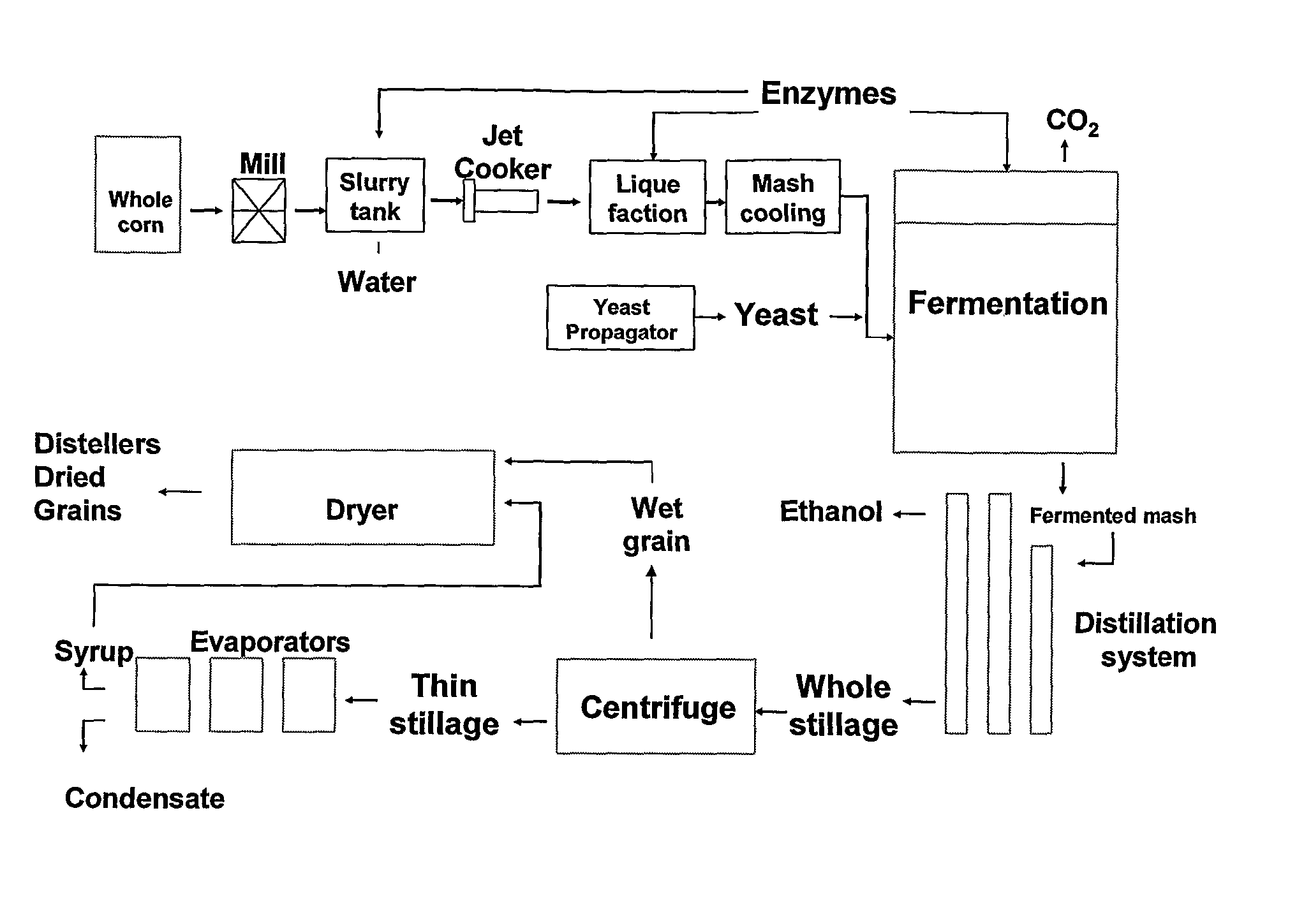
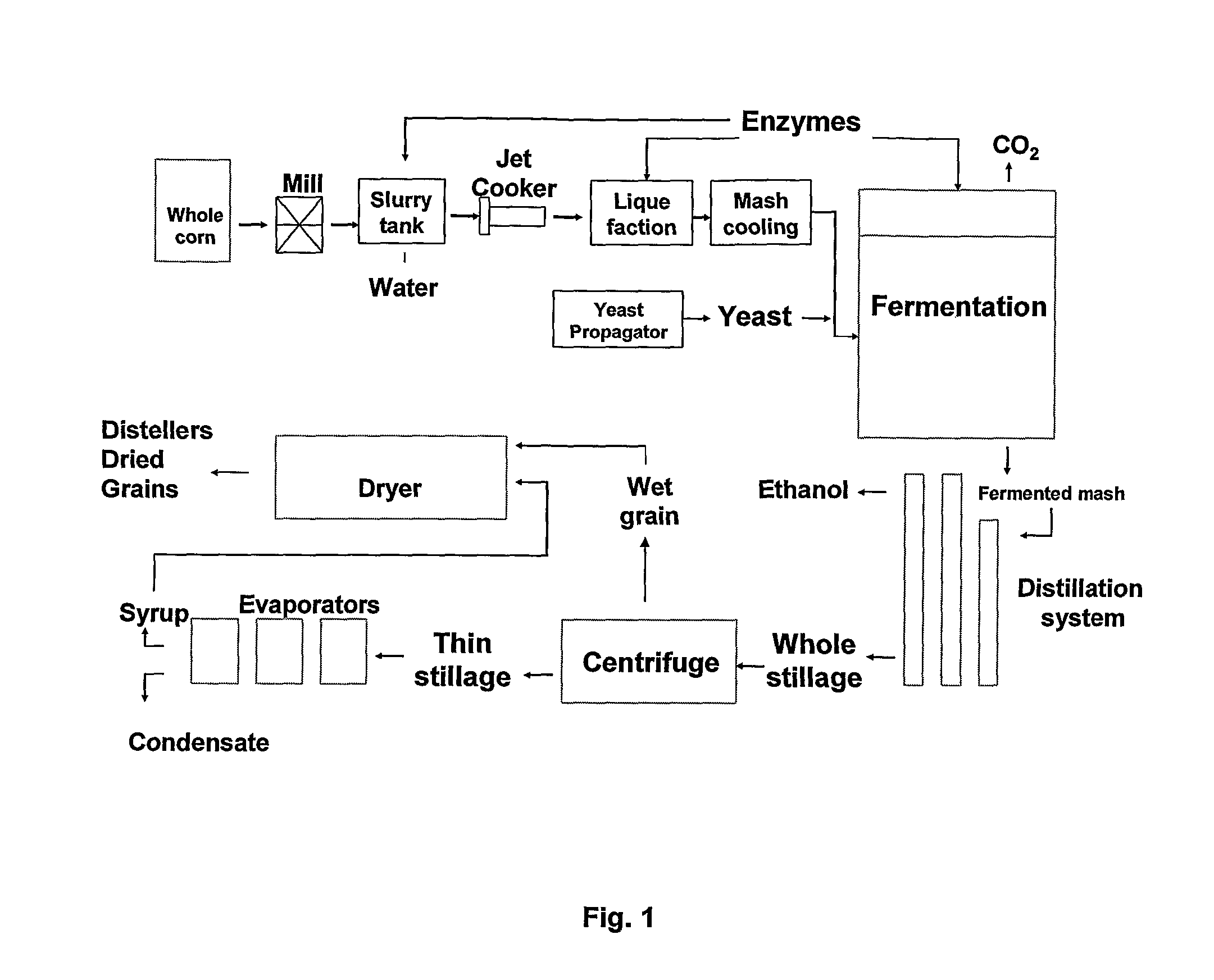
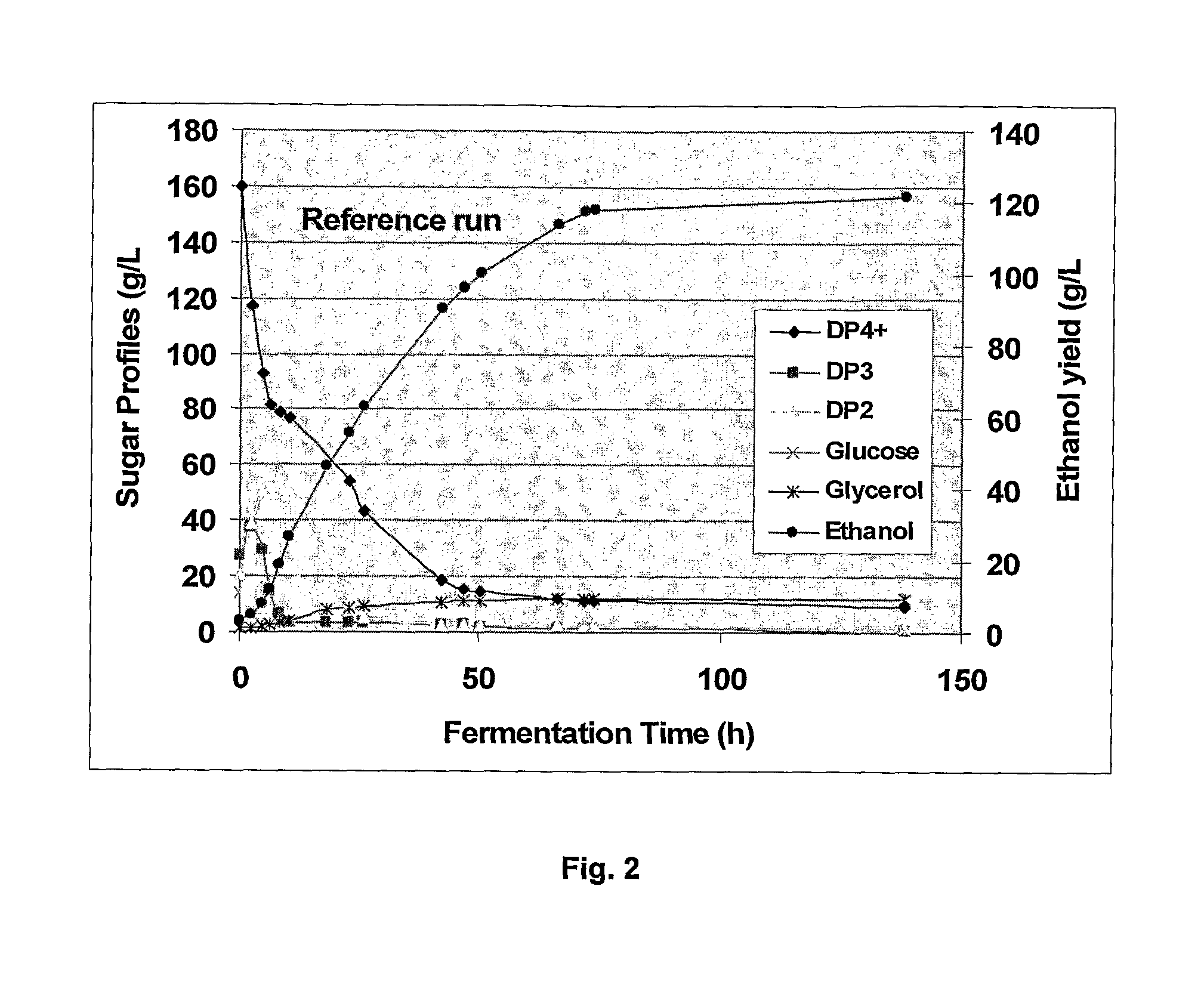
Process Of Producing A Fermentation Product
The invention relates to a process of producing a fermentation product, such as ethanol, from starch-containing material, including i) subjecting starch-containing material to an alpha-amylase, ii) subjecting the material obtained in step i) to an alpha-glucosidase and / or a maltose-generating enzyme, and iii) fermenting the material in the presence of a fermenting organism, such as yeast. Alternatively the invention relates to a process of producing a fermentation product from starch-containing material, preferably granular starch, which process comprises: a) subjecting starch-containing material to an alpha-glucosidase and optionally a glucose-generating and / or maltose-generating enzyme, and b) fermenting the material in the presence of a fermenting organism.
Owner:NOVOZYMES AS +1
Total flavone extract of abelmoschus manihot and preparing method of total flavone extract
The invention discloses the weight ratio of cotton-3'-glucosidase, meletin-3'-glucosidase and isoquercitrin in total flavone extract of abelmoschus manihot. The total flavone extract of abelmoschus manihot has definite effective ingredients, more stable quality, more completed effective ingredients, better and more stable curative effect, safety and reliability, simple preparing process and low cost, is suitable for industrial production, has better effective ingredient ratio, high content and high quality and solves the problem of large dose of abelmoschus manihot.
Owner:JIANGSU SUZHONG PHARM GRP CO LTD
Glucosidase/xylosidase difunctional cellulose degradation enzyme RuGBGX2 as well as coding gene and application thereof
ActiveCN102041251AHigh activityReduce complexityMicroorganism based processesEnzymesChemical industryCellulose
The invention relates to a novel beta glucosidase / xylosidase difunctional cellulose degradation enzyme RuGBGX2 as well as a coding gene and application thereof. The coding sequence of amino acid of the RuGBGX2 contains 18-755th sites of an SEQ ID NO 2 sequence. The RuGBGX2 is sourced from the rumen microorganism of yak from China, a novel coding gene of the beta glucosidase / xylosidase difunctional cellulose degradation enzyme RuGBGX2 is obtained by function screening and sequencing analysis on a rumen metagenome cosmid library and a subclone library. The beta glucosidase / xylosidase difunctional cellulose degradation enzyme provided by the invention can be widely applied to the degradation of cellulose and the fields such as cellulose biotransformation, chemical industry, spinning, foods, bioenergy, feed additives, medical industry and the like. By utilizing the difunctional enzyme RuGBGX2 to degrade wood fiber, the varieties of added enzymes can be reduced, and an enzymolysis process can be simplified.
Owner:FUDAN UNIV +1
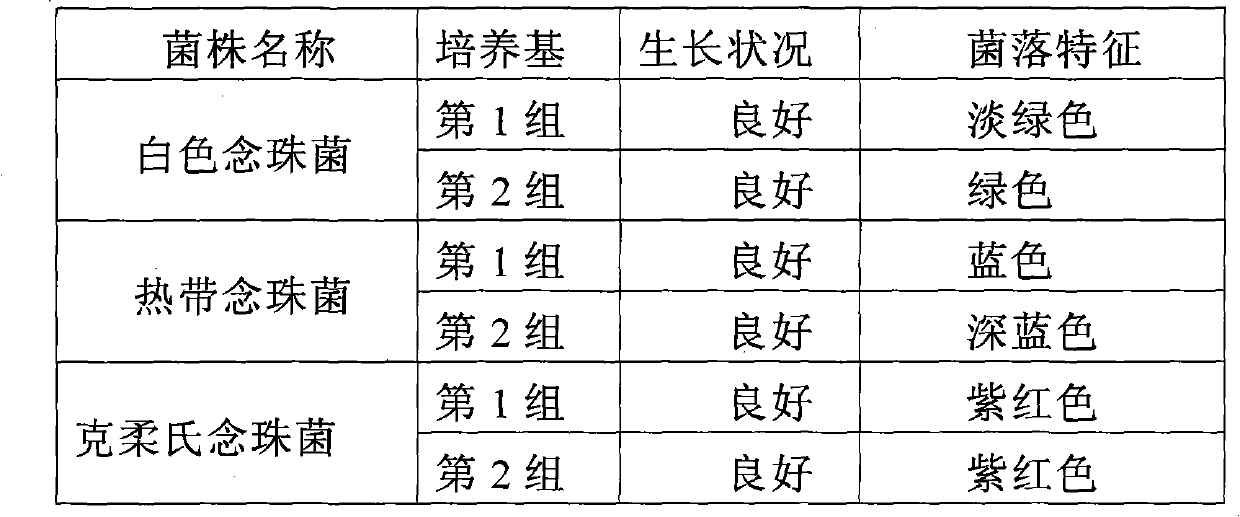
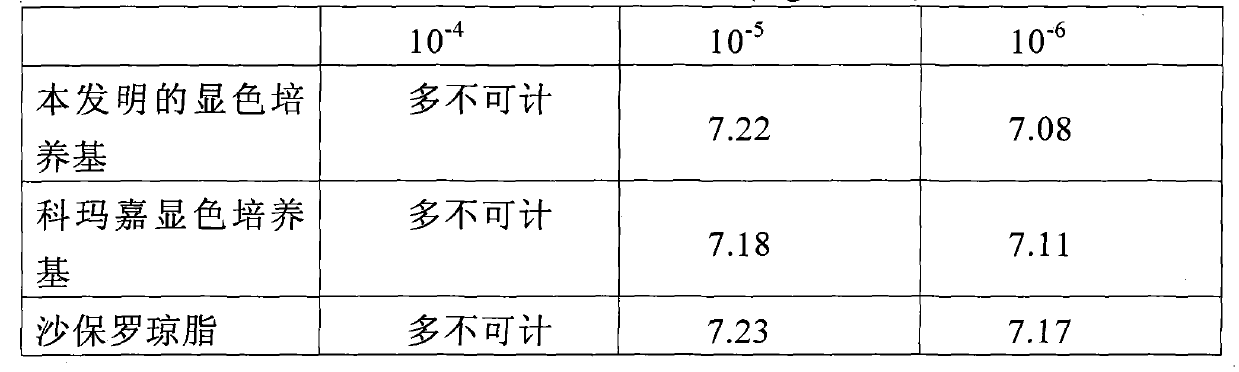
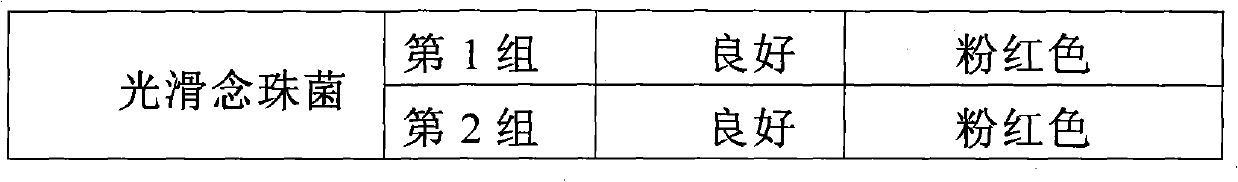
Candida chromogenic medium, detection kit and detection method
ActiveCN101948902AFast growthOvercome the disadvantage of not being able to distinguish the various species of CandidaMicrobiological testing/measurementMedical equipmentMedical microbiology
The invention relates to candida chromogenic medium, a detection kit and a detection method, belonging to the technical field of microbial diagnosis, in particular to the cultivation and identification of yeast in medical microbiology specimens, drugs and medical equipment sanitary inspection samples, public health surveillance samples and food (including cosmetics) sanitary inspection samples. The chromogenic medium of the invention is composed of basic medium, mixed chromogenic substrate and bacteriostat, wherein the mixed chromogenic substrate consists of aminocaproic glucosidase and alkaline phosphatase substrate, and candida specific enzyme is added into the mixed chromogenic substrate. The detection kit of the invention consists of the chromogenic medium, identification paper A containing enzyme substrate 5-bromo-4-chloro-3-indolyl-N-acetyl-beta-D-aminogalactose and identification paper B containing enzyme substrate 5-bromo-4-chloro-3-indolyl-beta-D- glycopyranoside. In the invention, the candida chromogenic medium and the detection kit have the advantages of low cost and simple configuration, and the method can be applied to the separation and identification of candida rapidly, simply and accurately.
Owner:BEIJING JUNLIKANG BIOTECHNOLOGY CO LTD
Cleaning compositions comprising transglucosidase
InactiveUS20080229514A1Efficient removalNon-ionic surface-active compoundsNon-surface-active detergent compositionsPolysaccharideEnzyme
Provided herein is a composition comprising: a) a transglucosidase enzyme; and b) a natural gum polysaccharide, wherein said natural gum polysaccharide is a substrate for said transglucosidase enzyme. A method of using a transglucosidase enzyme to a degrade natural gum polysaccharide is also provided. The composition and method may be employed in cleaning applications.
Owner:DANISCO US INC
Mulberry bark extract with glycosidase inhibiting function and preparation thereof
The invention discloses a white mulberry root-bark exact with the glucosidase inhibition function, a preparation method thereof and a quality control method. During the preparation of the white mulberry root-bark extract, extraction, concentration and centrifugalization are adopted, and the methods of three different types of anion-cation exchange resin columns, drying and so on are carried out to fully extract and highly concentrate the effective drugs; at the same time, the invention further provides the quality control method for carrying out the content measurement of the extract.
Owner:BEIJING WBL PEKING UNIV BIOTECH
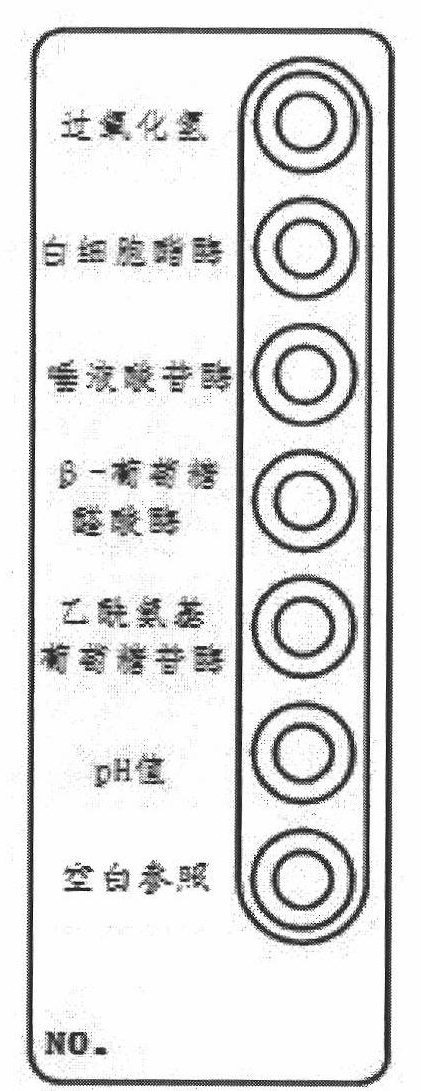
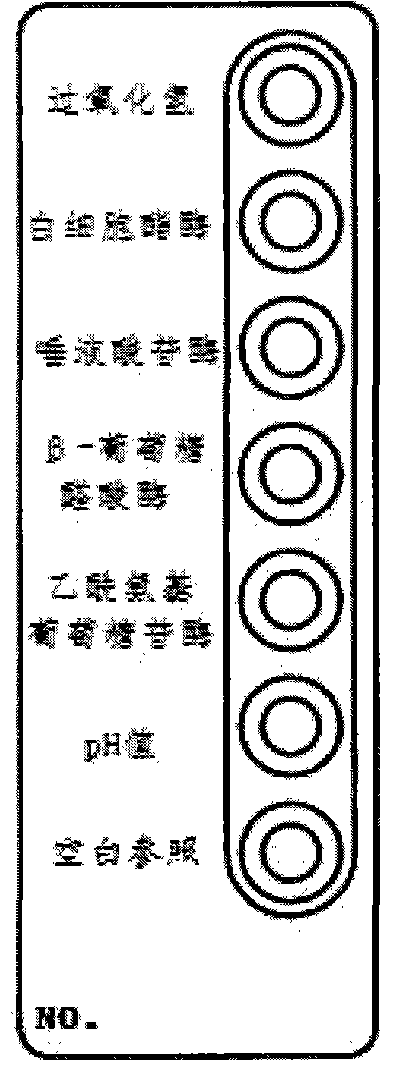
Vaginitis test kit and preparation method thereof
ActiveCN102321731AEasy to operateImprove accuracyMaterial analysis by observing effect on chemical indicatorMicrobiological testing/measurementBacterial vaginosisFacultative anaerobic organism
The invention discloses a vaginitis test kit which comprises a reaction device, a sampling test tube, a straw, joint test diluent, joint test chromogenic reagent, joint test stop solution, a user manual and a joint test colourimetric card, wherein a hydrogen peroxide reaction hole, a leukocyte esterase reaction hole, a sialic acid glucoside enzyme reaction hole, a beta- glucuronic acid enzyme reaction hole, a P glucosidase reation hole and a pH value hole; relevant reaction bases are arranged in the reaction holes; reach reaction base comprises a corresponding reaction substrate curing layer,a chromogenic promotional layer and a chemical inert carrier layer. The invention also provides a preparation method of the test kit and the preparation method of a novel chemical carrier. The test kit can be used for distinguishing bacterial vaginosis and vaginitis, and can further identify aerobic / anaerobic bacteria, facultative anaerobic bacteria and other flora in vaginal secretion. The method is simple and quick to operate, has high accuracy, and is applicable to clinical practice, particularly hospital outpatient practice.
Owner:JIANGSU BIOPERFECTUS TECH CO LTD
Recombinant thermoascus aurantiacus beta-glucosidase variants for production of fermentable sugars from cellulosic biomass
The present invention provides compositions and methods for the expression of recombinant β-glucosidase variants, as well as their use in the production of fermentable sugars from cellulosic biomass.
Owner:CODEXIS INC
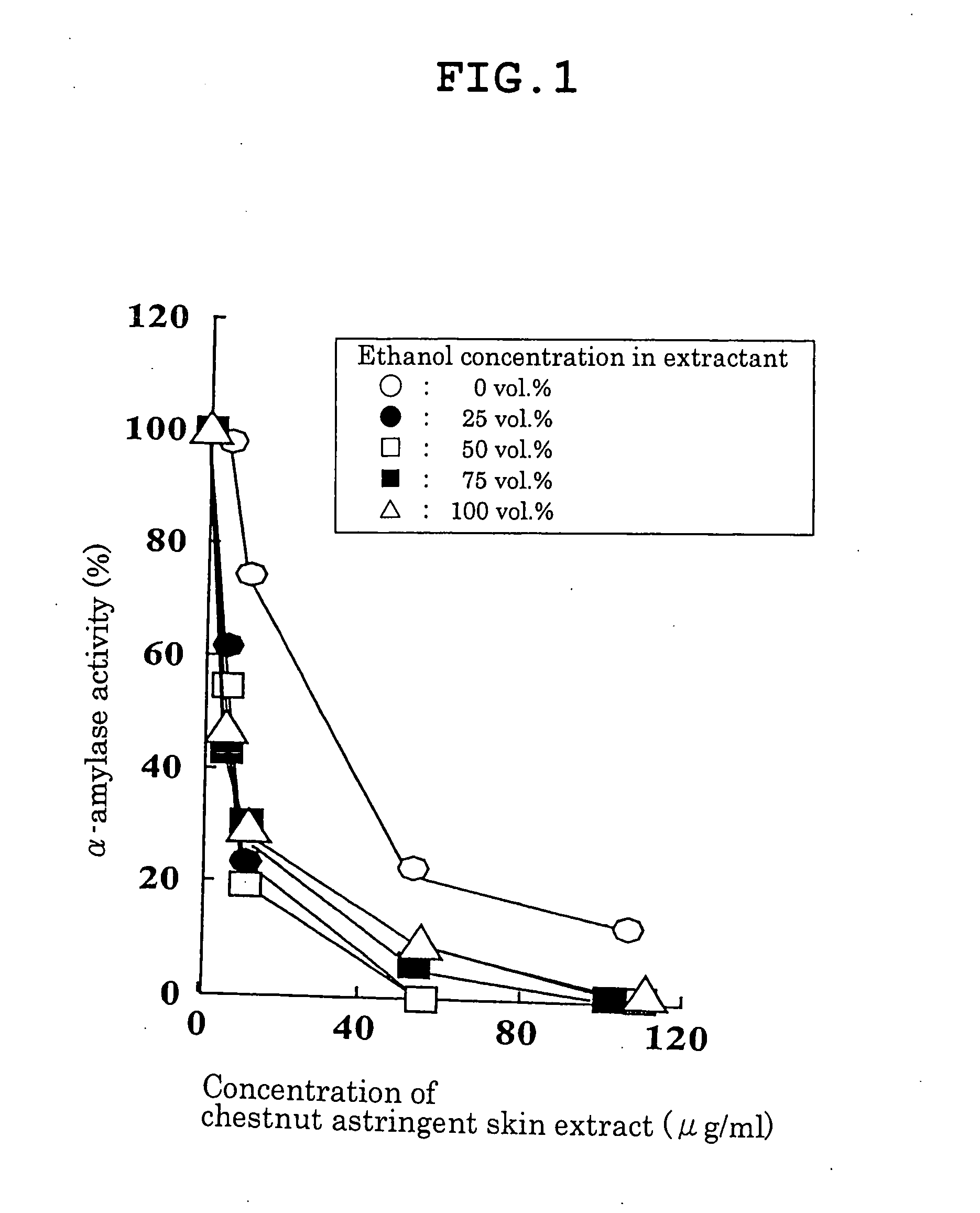
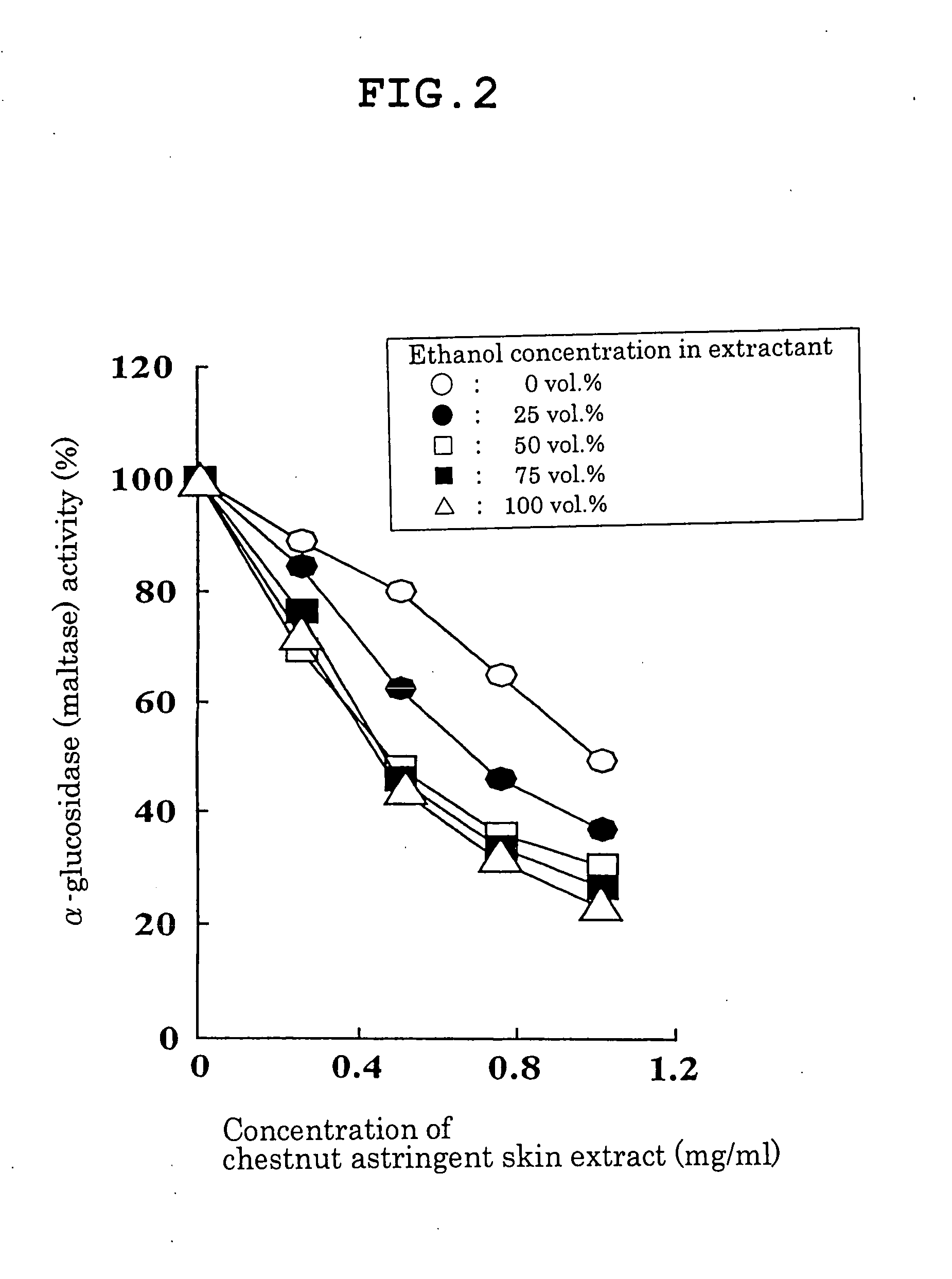
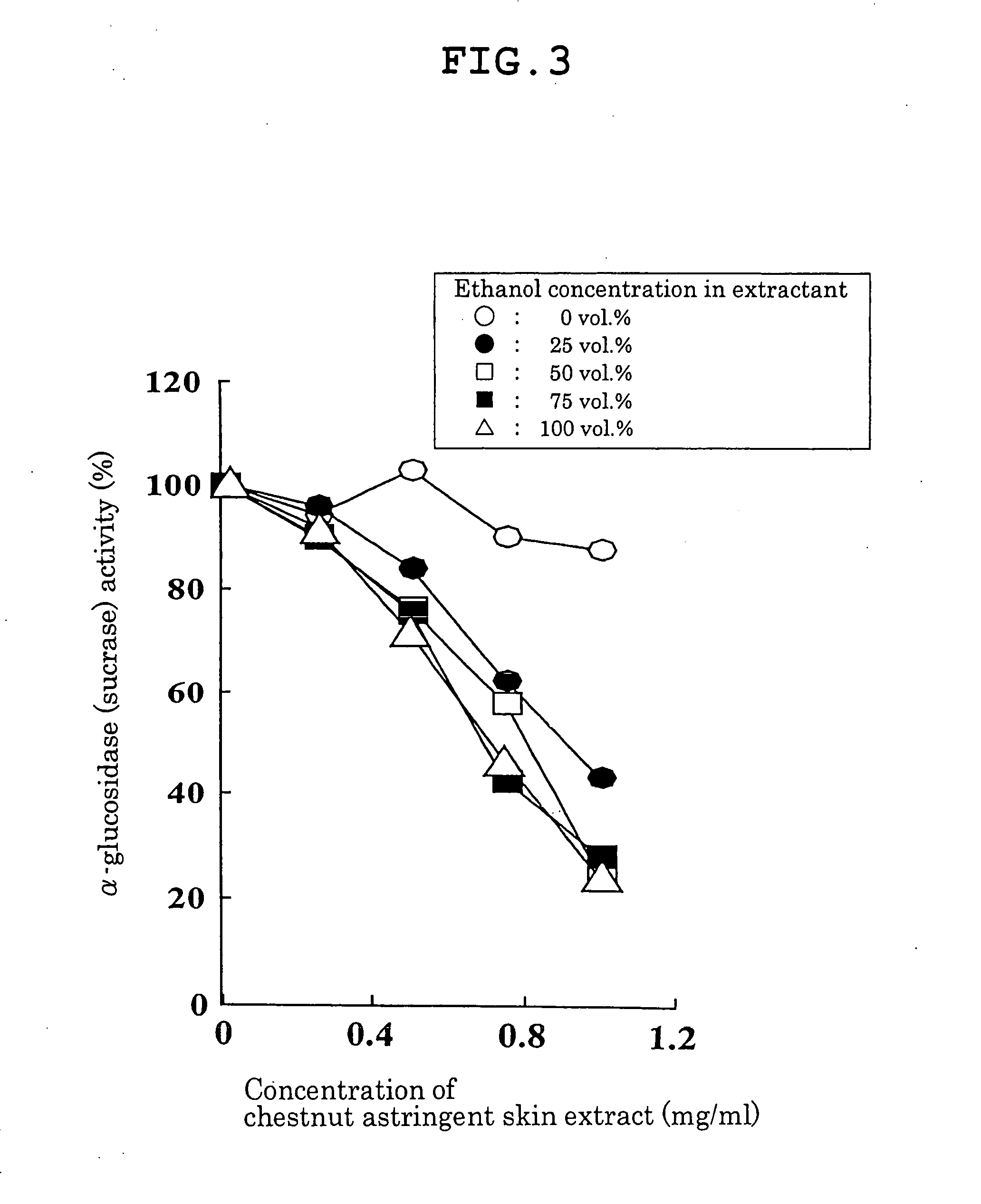
Carbohydrase Inhibitors Derived From Chestnut And Use Thereof
InactiveUS20070202205A1Stable supplyReduce digestion and absorptionBiocideSenses disorderAmylase inhibitorsBlood insulin
The present invention provides a plant-derived carbohydrase inhibitor, wherein the inhibitor is effective for preventing or alleviating diabetes, or preventing obesity, and foods, drinks, and medicines containing the same. The present invention is accomplished by use of an α-amylase inhibitor or an α-glucosidase inhibitor as a carbohydrase inhibitor that is extracted from astringent skins of a chestnut using ethanol or aqueous ethanol solution. The present invention can also be accomplished by adding the carbohydrase inhibitor to a food or medical composition as an active ingredient for delaying saccharide digestion or absorption, suppressing a rise in postprandial blood glucose levels or blood insulin levels, or preventing obesity.
Owner:TSUJITA TAKAHIRO +2
Agents for control of codling moth in fruit orchards
Owner:EFAL化学工业有限公司 +1
Method for preparing salidroside with enzymatic method
The invention provides a method for preparing salidroside with an enzymatic method. The method includes the specific steps that deep-eutectic solvents (DESs) synthesized by heating and stirring choline chloride and glycerin serve as reaction solvents, and the reaction solvents are biologically catalyzed and glycosylated into salidroside through beta-D-glucosidase, wherein the conversion rate of a substrate can be 30% or above (by glucose). According to the method, the adopted DESs are green and nontoxic, the solubility of the reaction substrate is high, and good biocompatibility is achieved. The preparing method has the advantages of being easy to operate, efficient, environmentally friendly, mild in condition, low in cost and the like.
Owner:SHANXI UNIV
Process for preparing inslin and oligofructose using xuelianguo fruits as raw material
The invention relates to a producing process of the inulin and the oligo fructose produced from the fruit of the snow lotus. The process is mainly that the fresh fruit of the snow lotus is cleaned, chipped, expressed juice, filtrated, dried, crushed characterized in that the slicing is dipped into the color fixative 1-3 hour and the inulin is purified by adding the 10% confected inulinase and the water and is fermented 48-72 hour in the aerobic atmosphere of the temperature of 20-30. The snow lotus inulin and the glucosidase fructose can be used for the health products, the food additive.The using of it as the health products has many merits of the low consumption of taking medicine, the good curative effect, taking conveniently, transporting and storing conveniently. So it can be added into the food such as the drink, the cake and the candy to form the functional food. The invention has some merits of the advance arts and crafts, the high quality.
Owner:昆明瑞鹏生态农业科技有限公司
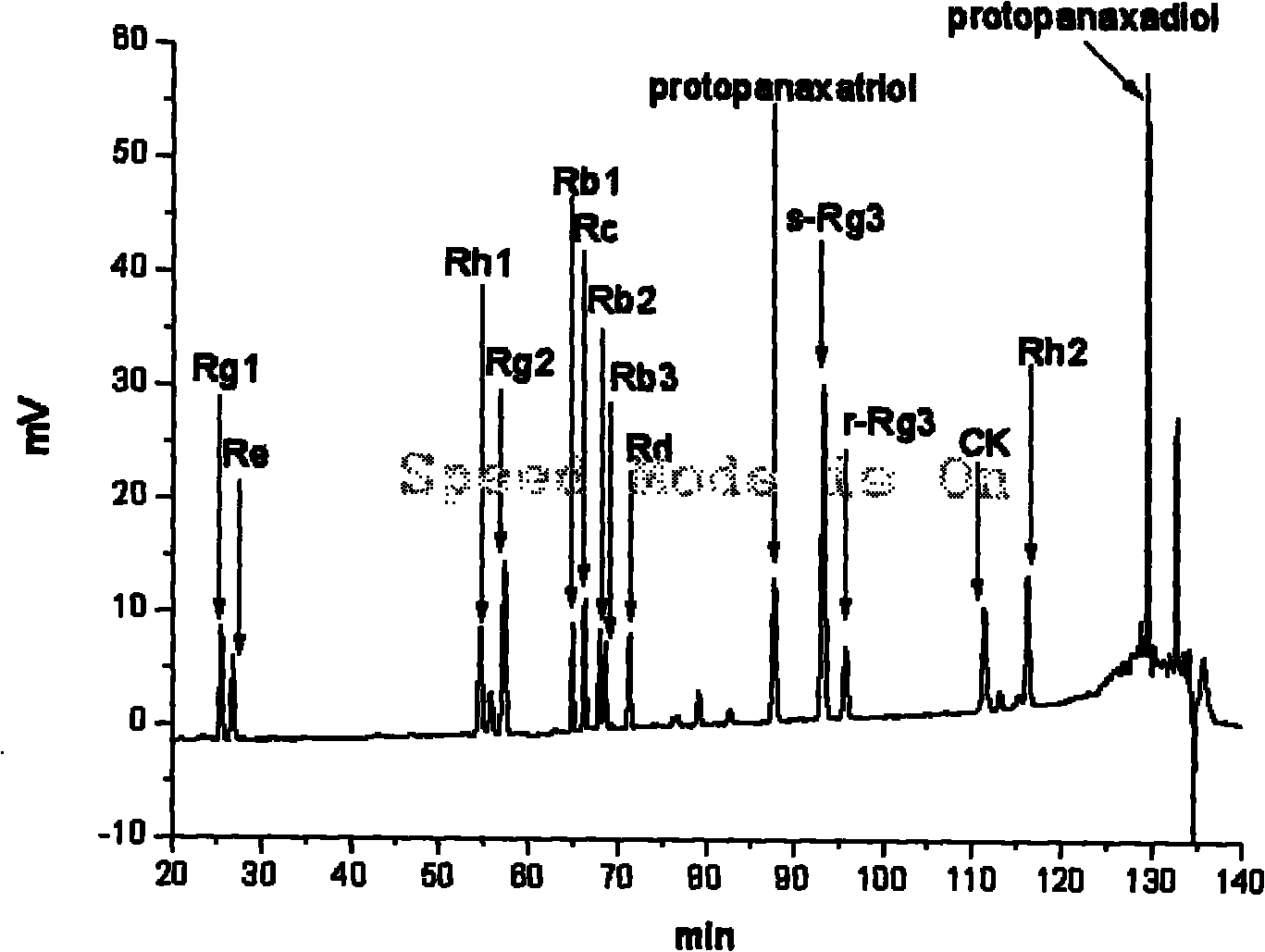
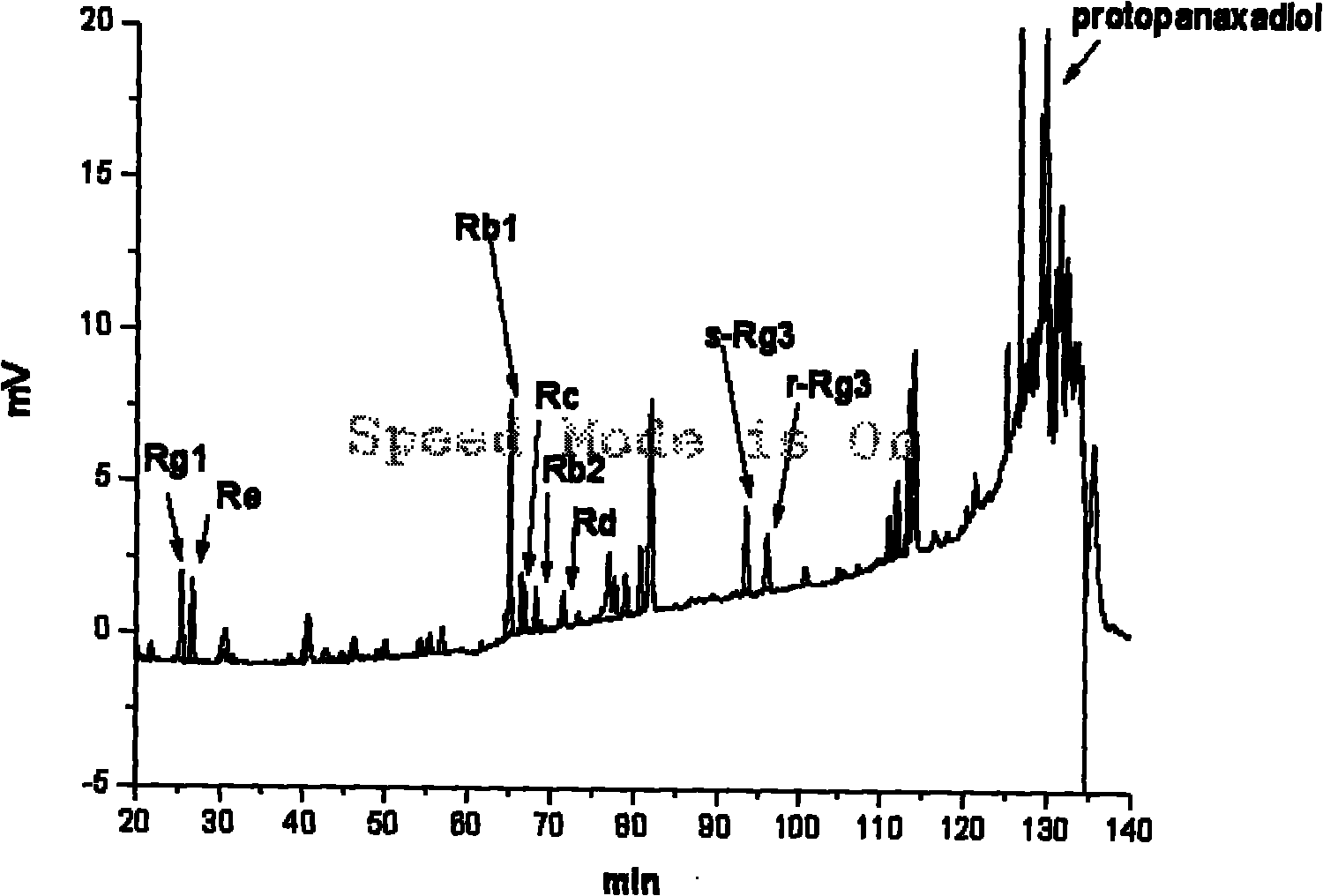
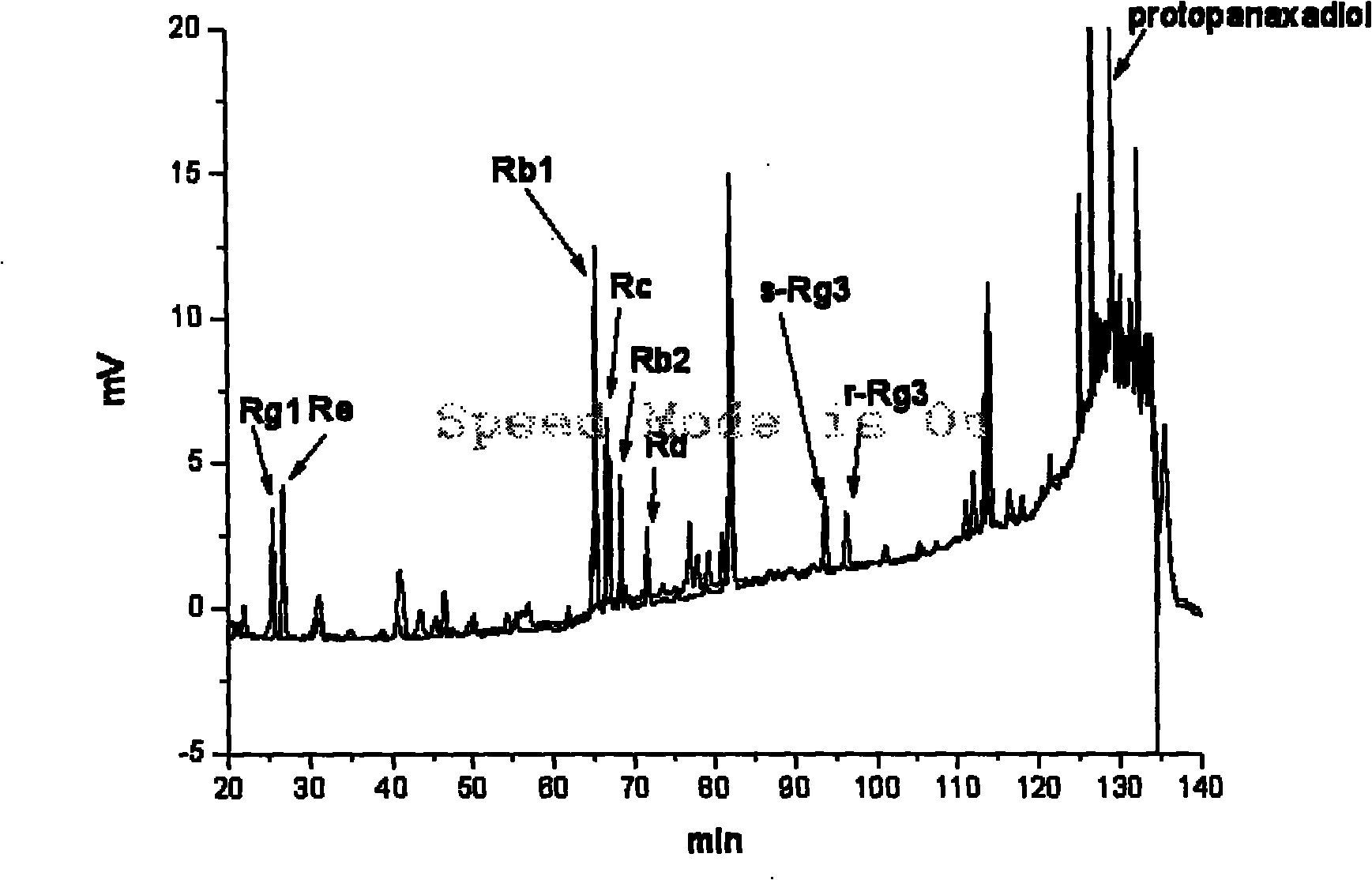
Preparation method of brown ginseng
ActiveCN101780128AHigh Rare Saponin ContentImprove efficiencyPlant ingredientsTime conditionAlglucerase
The invention discloses a preparation method of brown ginseng, which comprises the following steps of: hydrolyzing the crude raw materials of fresh ginseng, sun-dried ginseng, red ginseng, ginseng stems, ginseng leaves, ginseng flowers, ginseng fruits and the like by beta-D-glucosidase, conducting high-temperature steam treatment under appropriate heating temperature and time conditions, and heating and drying to obtain the ginseng product. The ginseng product prepared by using the preparation method has the advantages of high content of rare saponin, less loss of total saponin, complete product configuration and the like, and meanwhile, the method has simple process, high efficiency and low cost.
Owner:吉林玉参医药科技有限公司
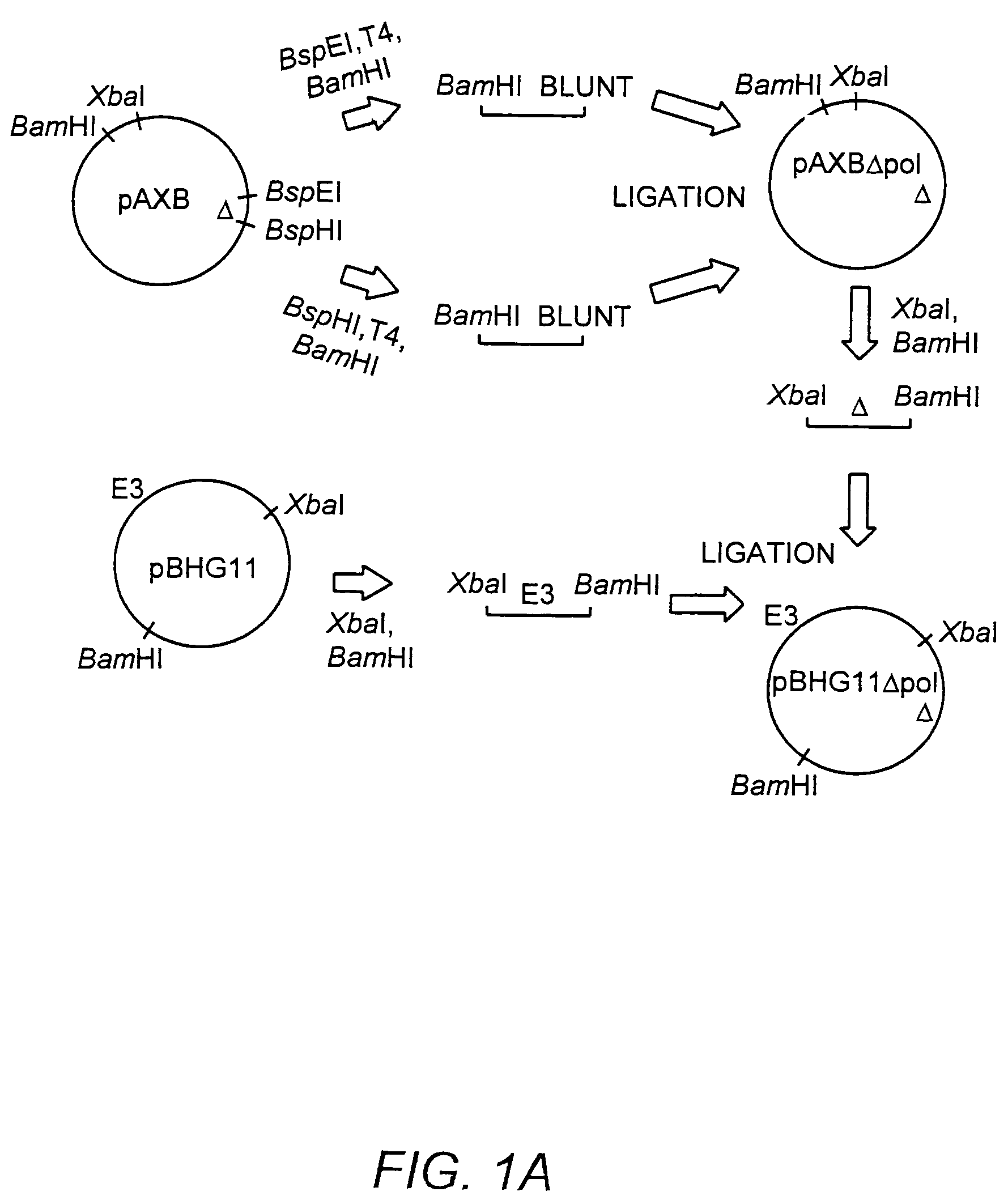
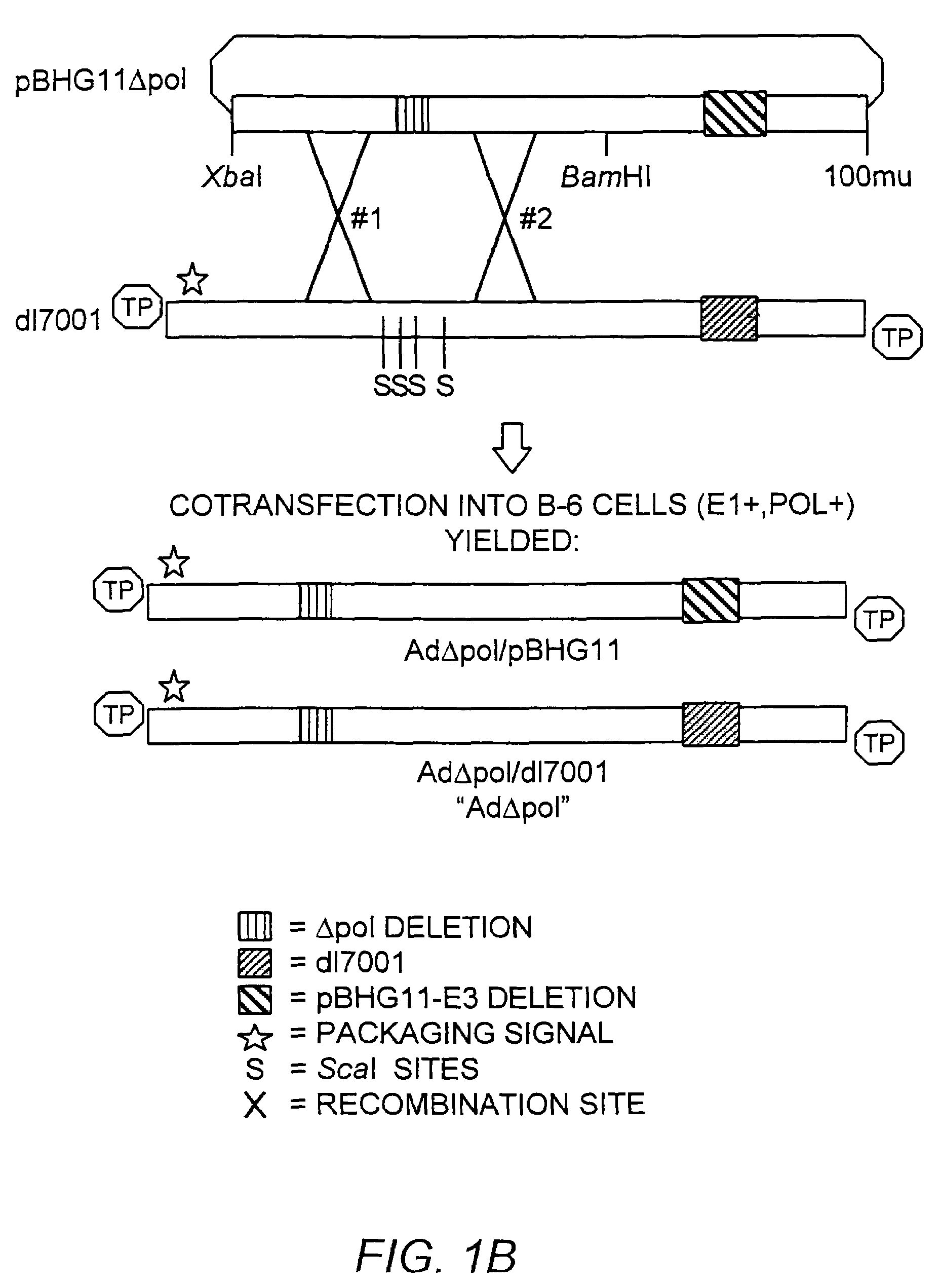
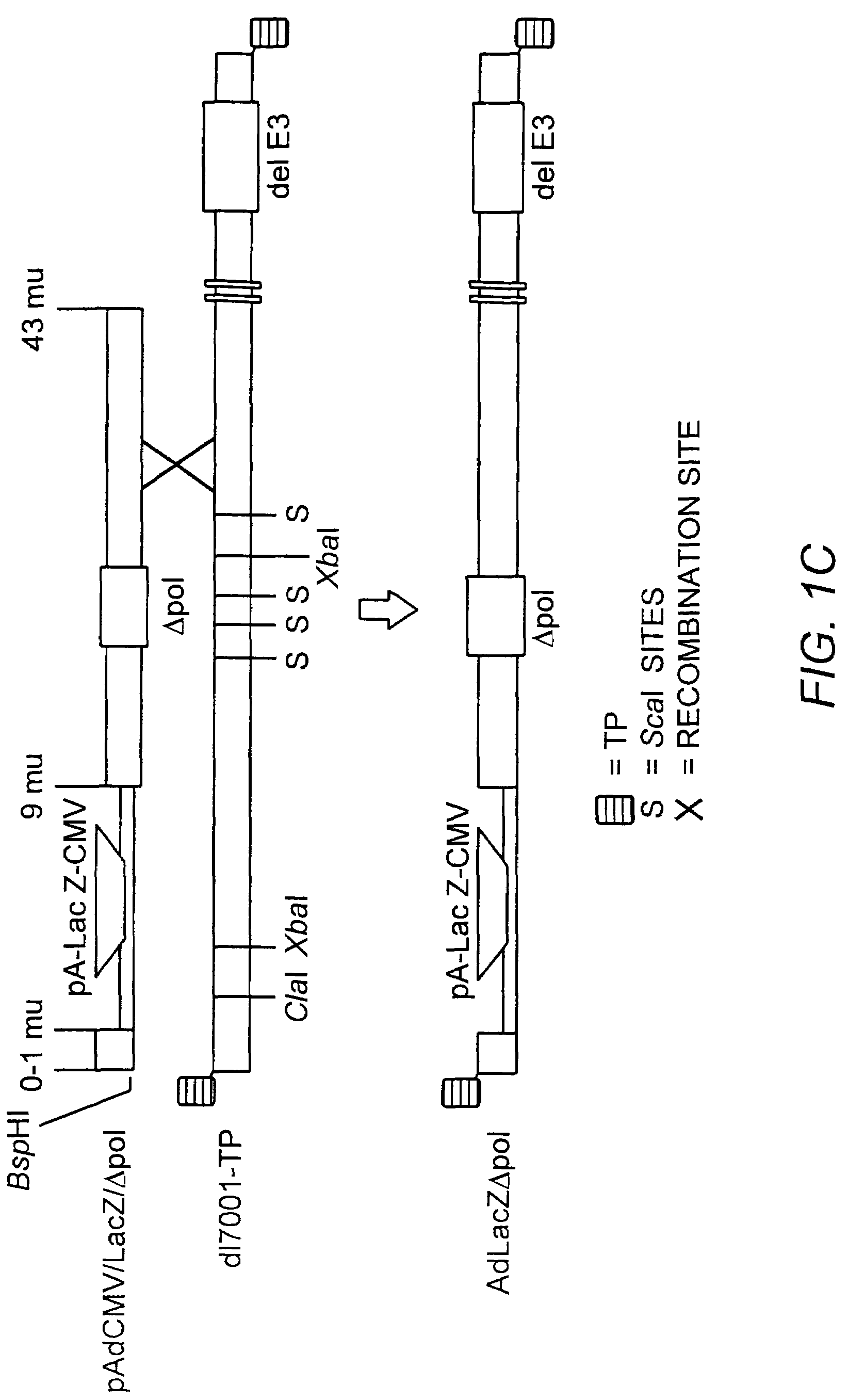
Deleted adenovirus vectors and methods of making and administering the same
The present invention provides deleted adenovirus vectors. The inventive adenovirus vectors carry one or more deletions in the IVa2, 100K, polymerase and / or preterminal protein sequences of the adenovirus genome. The adenoviruses may additionally contain other deletions, mutations or other modifications as well. In particular preferred embodiments, the adenovirus genome is multiply deleted, i.e., carries two or more deletions therein. The deleted adenoviruses of the invention are “propagation-defective” in that the virus cannot replicate and produce new virions in the absence of complementing function(s). Preferred adenovirus vectors of the invention carry a heterologous nucleotide sequence encoding a protein or peptide associated with a metabolic disorder, more preferably a protein or peptide associated with a lysosomal or glycogen storage disease, most preferably, a lysosomal acid α-glucosidase. Further provided are methods for producing the inventive deleted adenovirus vectors. Further provided are methods of administering the deleted adenovirus vectors to a cell in vitro or in vivo.
Owner:DUKE UNIV
Increased production of secreted proteins by recombinant eukaryotic cells
Owner:DANISCO US INC
Transglucosidase and its preparation and immobilization method
ActiveCN102296032AIncrease enzyme activityEasy to realize industrializationFungiTransferasesIsomaltooligosaccharideEngineering
The invention relates to transglucosidase, its preparation method and a immobilization method, which belong to the fields of enzyme engineering and fermentation engineering. More specifically, the invention provides the following processes: 1) Aspergillus niger BLB-16 is screened from soil as an original strain, processes of mutation treatment, optimization and screening are performed to obtain an optimized bacterial strain (BLB-28) for fermenting; 2) a fermentation broth is carried out a heat sterilization to obtain transglucosidase liquid; 3) transglucosidase liquid is performed a nanofiltration to concentrate; 4) processes of resin adsorption, sodium alginate entrapment, immobilization by a glutaraldehyde cross-linking method are carried out for preparing immobilized enzymes. The prepared transglucosidase is suitable for an application in the industrial fields such as foodstuff, medicine, feed and the like, and used for producing isomaltose hypgather.
Owner:BAOLINGBAO BIOLOGY
Methods of use and nutritional compositions of Touchi Extract
InactiveUS8815312B2Improve blood sugar controlDelaying the appearance of glucose in the bloodBiocideAntiviralsPhysiologyConstipation
Disclosed is a method and composition for nutritional compositions containing glucosidase inhibitors, and more specifically Touchi Extract and its uses in the treatment of many disorders. These disorders include diabetes, hyperlipidemia, obesity, Metabolic syndrome / Syndrome X, COPD, malabsorption, Crohn's disease, diarrhea, constipation, irritable bowel syndrome, human immunodeficiency virus, cystic fibrosis, non-alcoholic steatohepatitis, polycystic ovarian syndrome including associate infertility, and erectile dysfunction. Further, glucosidase inhibitors, and more specifically Touchi Extract can be used to aid healing in critical care patients and for general wound healing. Additionally, glucosidase inhibitors, including Touchi Extract can be used to enhance athletic performance.
Owner:NESTEC SA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com