Method for preparing salidroside with enzymatic method
A salidroside, enzymatic preparation technology, applied in the field of enzymatic preparation of salidroside, can solve the problems of not much improvement in synthesis results, poor enzyme activity and stability, disadvantages, etc., to achieve improved thermal stability, process The effect of mild conditions and low volatility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] A. Synthesis of deep eutectic solvent: Accurately weigh 13.97g of choline chloride, measure 14.56mL of glycerin, mix them into a 500mL round bottom flask, heat and stir in a collector magnetic stirrer at a temperature of 80-100°C , the speed is 300rpm. After 10 h of reaction, a uniform transparent liquid was formed, and about 30 mL of DES was obtained.
[0030] B. Preparation of β-D-glucosidase immobilized on chitosan microspheres: Weigh 0.5 g of chitosan and dissolve it in 100 mL of 2% glacial acetic acid to prepare chitosan colloidal solution, and ultrasonically remove air bubbles. Use a syringe to draw an appropriate amount of the above-mentioned chitosan solution, drop it into the coagulation liquid, the composition of the coagulation liquid is 7.5% NaOH solution / 95% ethanol (v / v) = 4:1, and form microspheres with a size of about 1.5mm. , added to 3% glutaraldehyde solution, oscillating and cross-linking at 50°C for 2h, washing away excess glutaraldehyde, adding β-...
Embodiment 2
[0033] A. With embodiment 1.
[0034] B. With embodiment 1.
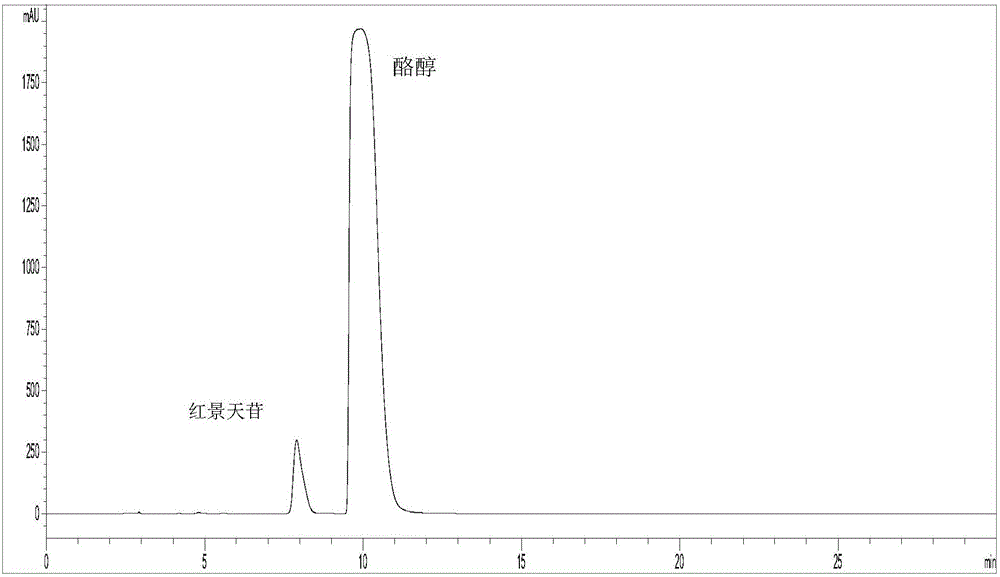
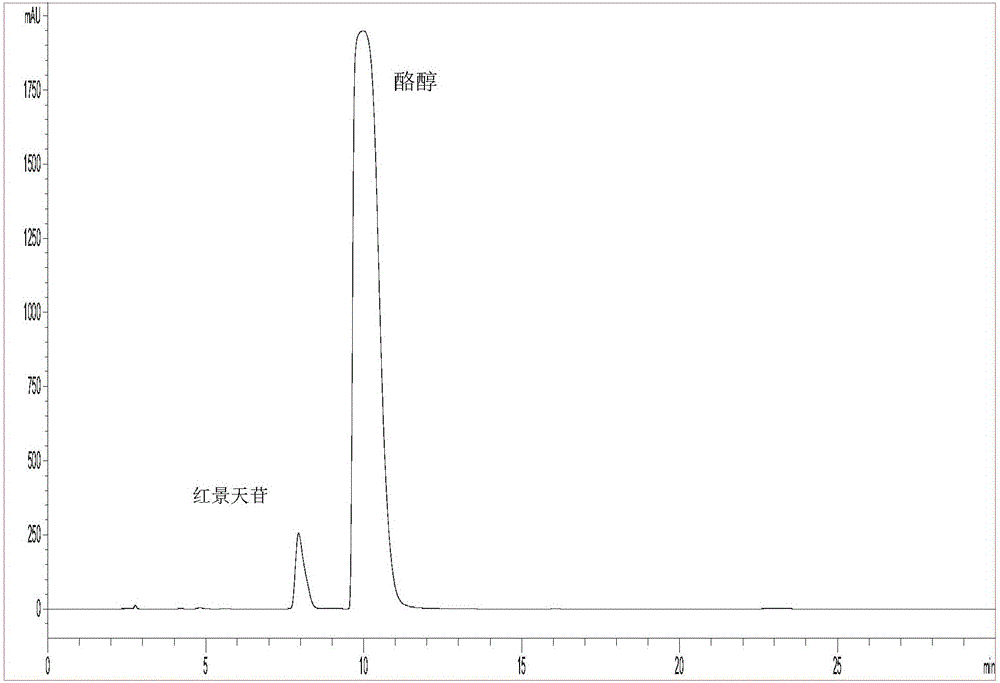
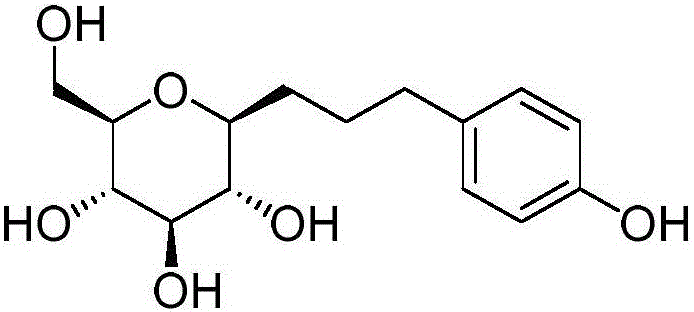
[0035] C. Enzyme-catalyzed synthesis of salidroside: Accurately weigh 1.8g of anhydrous D-glucose and 11.06g of tyrosol, and stir to fully dissolve in 180mL DES, then add 20mL of citric acid at pH 5.8 pre-incubated at 45°C in sequence - Disodium hydrogen phosphate buffer solution, 10000U chitosan microsphere immobilized β-D glucosidase, mix well. The above system was heated to 45° C., kept warm and stirred for 120 h. After the reaction, the reaction solution was filtered out and diluted with an equal volume of methanol. The conversion rate of the substrate (glucose meter) was detected by HPLC to reach 32.9%.
Embodiment 3
[0037] A. With embodiment 1.
[0038] B. With embodiment 1.
[0039] C. Enzyme-catalyzed synthesis of salidroside: Accurately weigh 1.8g of anhydrous D-glucose and 12.44g of tyrosol, stir to dissolve them in 140mL of DES, then add 60mL of citric acid at pH5.8 pre-incubated at 45°C in sequence - Disodium hydrogen phosphate buffer solution, 7000U chitosan microsphere immobilized β-D glucosidase, mix well. The above system was heated to 50° C., kept warm and stirred for 120 h. After the reaction, the reaction liquid was filtered out and diluted with an equal volume of methanol. The conversion rate of the substrate (glucose meter) was detected by HPLC to reach 31.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com