BVDV (Bovine Virus Diarrhea Virus) SMU-Z6/1a/SC/2016 isolate and application thereof
A technology of bovine viral diarrhea and SMU-Z6, which is applied in the field of bioengineering to achieve the effects of good immunogenicity, strong pertinence and broad market application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
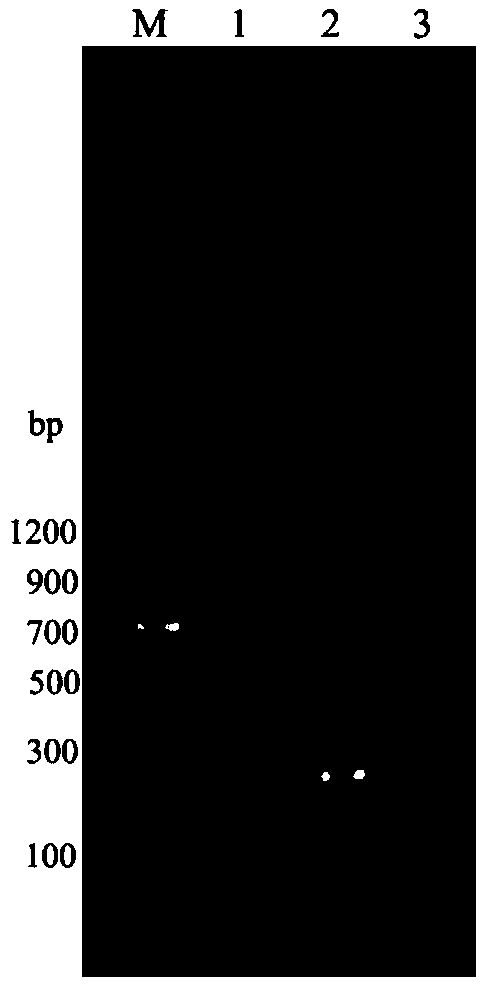
[0031] The isolation and identification of embodiment 1 bovine viral diarrhea virus
[0032] 1.1 Isolation of bovine viral diarrhea virus
[0033] First observe the morbidity of the cattle herd, and the inventor collects a cattle feces sample initially diagnosed as diarrhea. Stool samples were diluted 1:5 with PBS (100 U / mL penicillin, 100 g / mL streptomycin). Centrifuge at 3000r / min for 10min, and the obtained supernatant is filtered and sterilized by a 0.22μm filter membrane to obtain a disease material treatment solution.
[0034] Inoculate the above-mentioned disease material treatment solution on a vigorously growing monolayer of MDBK cells on a 6-well culture plate, inoculate 0.5 mL per well, and set up negative control wells at 37°C with 5% CO 2 Incubate for 1 hour in the incubator, discard the inductive solution, add 2 mL of maintenance solution (DMEM with 1% fetal bovine serum), at 37°C containing 5% CO 2 Cultivate in an incubator, observe the cytopathic changes eve...
Embodiment 2B
[0052] The development of embodiment 2BVDV inactivated vaccine
[0053] 2.1 Virus reproduction
[0054] Take the MDBK monolayer cells in a good growth state, wash them twice with Hanks, inoculate the cell culture medium of the above-mentioned vaccine candidate strain SMU-Z6 / 1a / SC / 2016 with good immunogenicity into the MDBK cells, add 1% fetal bovine Serum in DMEM cell maintenance solution at 37°C with 5% CO 2 Cultivate in an incubator, and when the cytopathic rate is about 80%, harvest the virus culture medium, freeze and thaw 3 times, and centrifuge at 4°C at high speed to obtain the virus supernatant as the virus for the vaccine.
[0055] 2.2 Preparation of virus inactivated vaccine
[0056] The cell culture supernatant of the vaccine candidate strain SMU-Z6 / 1a / SC / 2016 with good immunogenicity above was fully mixed with β-propiolactone with a final concentration of 0.5%, and shaken at 37°C. Bed, inactivation treatment 6h. Two copies of the inactivated virus solution were...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com