Classical swine fever E2 subunit vaccine and application thereof
A subunit vaccine, swine fever technology, applied in applications, medical preparations containing active ingredients, viral peptides, etc., can solve the problems of reduced safety and effectiveness, and achieve stable effective antigen quantity, good safety, antigenic high purity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1 preparation of classical swine fever virus E2 protein
[0030] 1) Optimization and synthesis of classical swine fever virus E2 gene
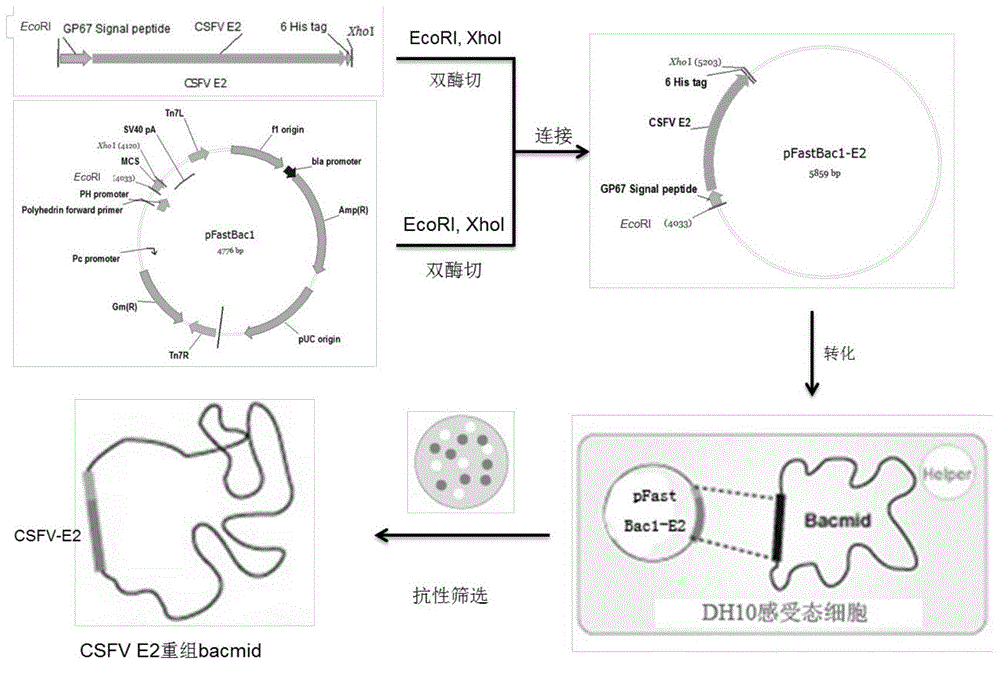
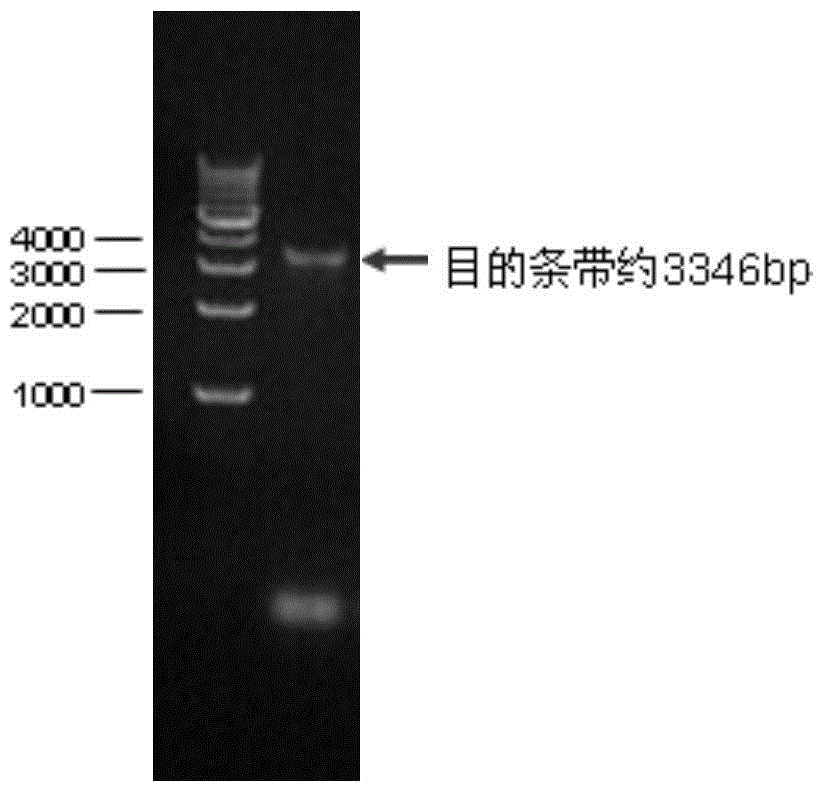
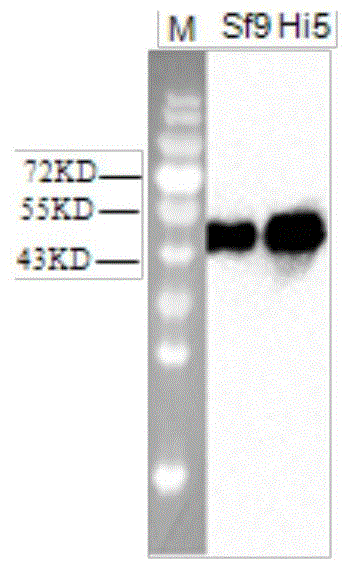
[0031] Referring to the E2 gene of classic strains and popular strains at home and abroad, select the classical swine fever virus strain of genotype 2.1 (gene sequence is Classical swine fever virus strain Zj0801, complete genome GenBank: FJ529205.1) according to the codon preference in insect cells The E2 gene of classical swine fever virus was optimized and handed over to the company for synthesis. The artificially synthesized genes include: GP67 signal peptide nucleic acid sequence (129bp), partial sequence of classical swine fever virus E2 gene (1011bp), 6His tag sequence (18bp) and fragment 5 EcoR I restriction site (6bp) at the 'end and Xho I restriction site (6bp) at the 3' end of the fragment (see figure 1 ) (shown in SEQ ID No.2).
[0032] 2) Cloning of the gene:
[0033] The artificially synthesized exogenous DNA...
Embodiment 2
[0041] Example 2 Preparation of classical swine fever virus E2 protein subunit vaccine
[0042] E2 protein of classical swine fever virus is emulsified with 201R to make vaccine: preparation of adjuvant, 201R adjuvant (121°C, 15pa, 20min) is sterilized, protein antigen and adjuvant are mixed 1:1, emulsified at 350rpm for 5min, namely The recombinant subunit vaccine of classical swine fever E2 was prepared.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com