Fully-human-derived anti-HCV (hepatitis C virus) neutralizing antibody-TRN1001
An antibody and whole-human technology, applied in the fields of cellular immunology and molecular biology, to prevent repeated HCV infection and reduce adverse reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Example 1 Preparation of fully human monoclonal neutralizing antibody TRN1001 against HCV
[0065] 1. PBMC Isolation and Single Memory B Cell Sorting
[0066] After cell counting, cells were sorted from PBMC by flow cytometry. First, cell debris, adherent cells and dead cells were removed, and CD3- / CD14- / CD16- / IgM- cells were obtained by fluorescent antibody staining, and CD235a-expressing cells were selected. / IgD- / CD20+ B cells, CD27ALL memory B cells were circled, and E2 double-fluorescence-labeled target cells were obtained with antigens specifically labeled with fluorescein.
[0067] 2. Single-cell RT-PCR isolation of antibody variable region genes
[0068] 1) Reverse transcription (RT) of single-cell RNA: Add 0.5 μM constant region primers and Superscript IV reverse transcriptase ( Invitrogen, Carlsbad, CA), positive and negative controls were set at the same time; reverse transcription PCR conditions: 55°C for 60min, cooled to 4°C. The product cDNA is stored a...
Embodiment 2
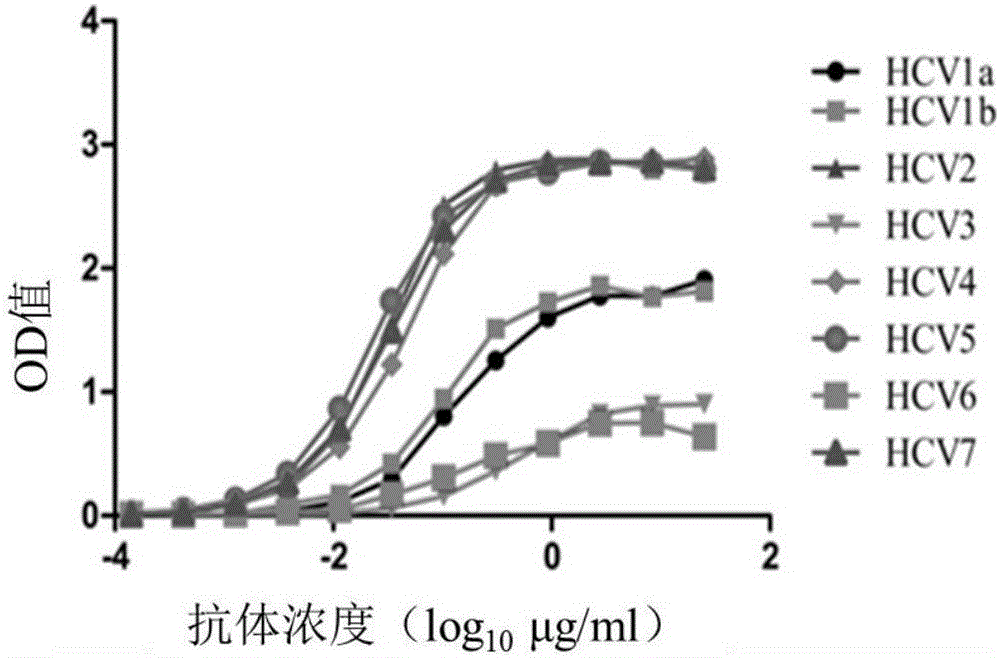
[0079] Example 2 TRN1001 Antibody Binding Activity Detection
[0080] 1. ELISA determination of binding activity
[0081] Steps: using different HCV envelope glycoproteins as antigens, and diluting the antigens to a concentration of 100 ng / ml with the coating solution, coating them on an ELISA 96-well plate, 100 μl per well, and overnight at 4°C. The blocking solution was blocked for 2 hours at 37°C. Add the primary antibody after blocking, the initial concentration of TRN1001 is 25 μg / ml, 3-fold serial dilution, the volume of each well is 100 μl, and incubate at 37°C for 1 hour. At the same time, HCV positive patient serum is used as a positive control, and rabies antibody is used as a negative control. HRP-labeled goat anti-human IgG (diluted 1:2000) was used as a secondary antibody and incubated at 37°C for 1 hour. Add 100 μL / well of substrate chromogenic solution (TMB), place at 37°C in the dark for 5 min, stop the reaction with 2M sulfuric acid, and perform colorimetry ...
Embodiment 3
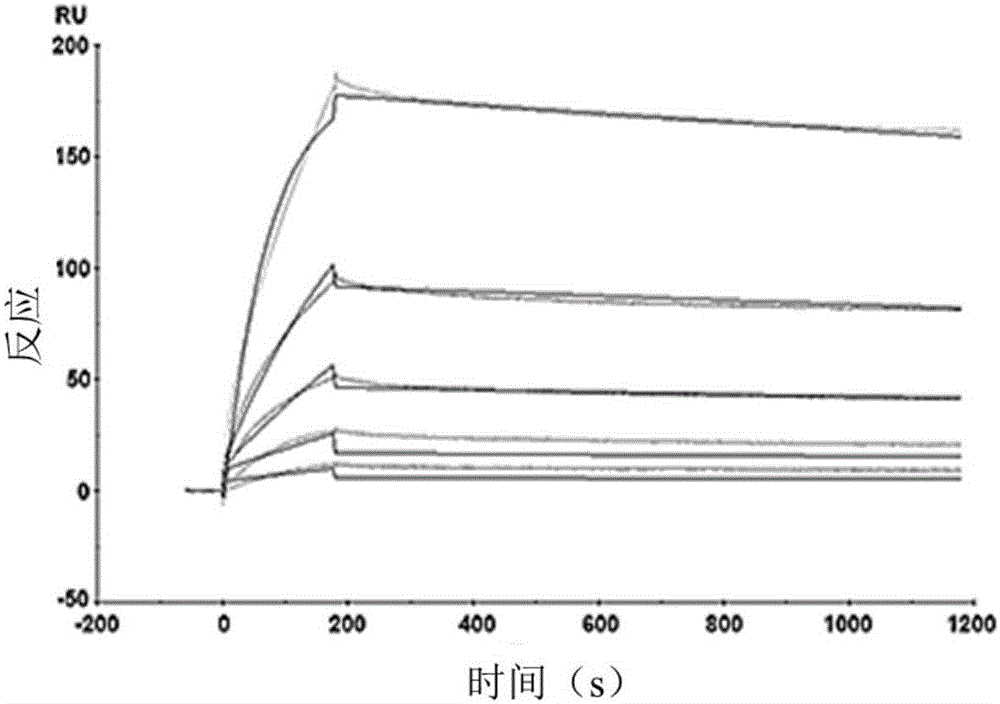
[0086] Example 3 TRN1001 Antibody Affinity Activity Detection
[0087] CM5 chip-coupled capture molecules: Goat anti-human IgG antibodies were immobilized on the CM5 sensor chip as capture molecules, and coupled to the gold film surface of the CM5 chip according to the operation of the coupling kit. The dextran surface of the chip was activated with EDC and NHS, the coupling amount was determined by the injection time, and finally the remaining activated groups on the surface were blocked with ethanolamine. Capture molecule capture ligand on CM5 chip: The prepared fully human anti-HCV neutralizing antibody is used as the ligand, and the signal value obtained by calculation is used to determine the injection concentration and contact time of the monoclonal antibody. Affinity and kinetics analysis of the binding of monoclonal antibody TRN1001 to HCV-E2 protein (antigen): HCV-E2 protein strain was diluted with HBS-EP buffer as the analyte, and the analyte flowed through the chip ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| affinity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com