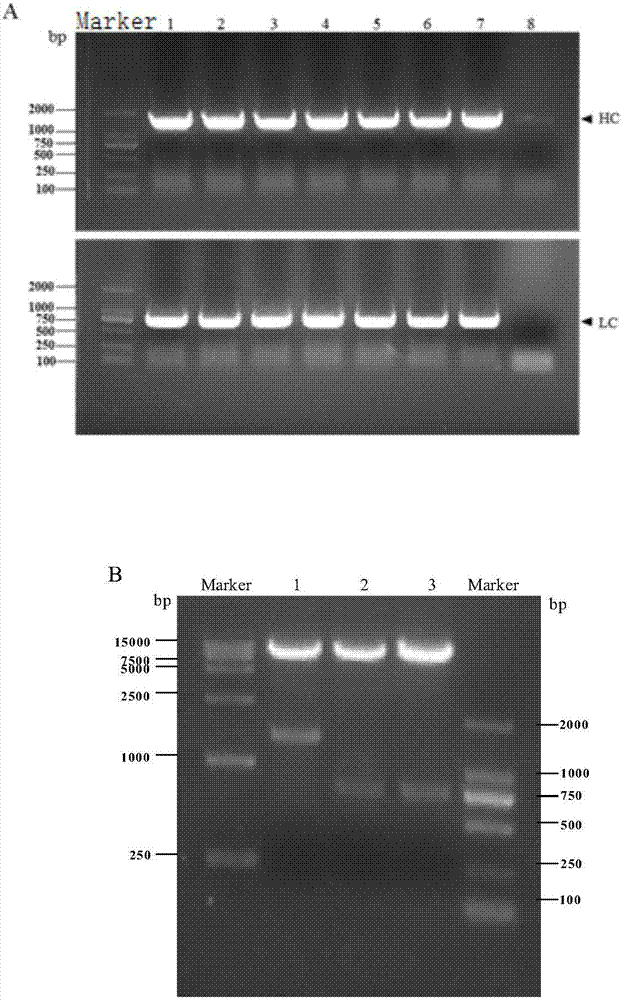
Recombinant swine fever virus E2 protein swine source monoclonal antibody and preparation method and application thereof
A monoclonal antibody, swine fever virus technology, applied in antiviral immunoglobulins, biochemical equipment and methods, viruses/phages, etc., can solve problems such as instability, hybridoma cells cannot be stored for a long time, and antibody cell viability declines.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1 Preparation of recombinant swine fever virus E2 protein porcine monoclonal antibody
[0043] 1. Materials and methods
[0044] 1.1 Cells, viruses and plasmids
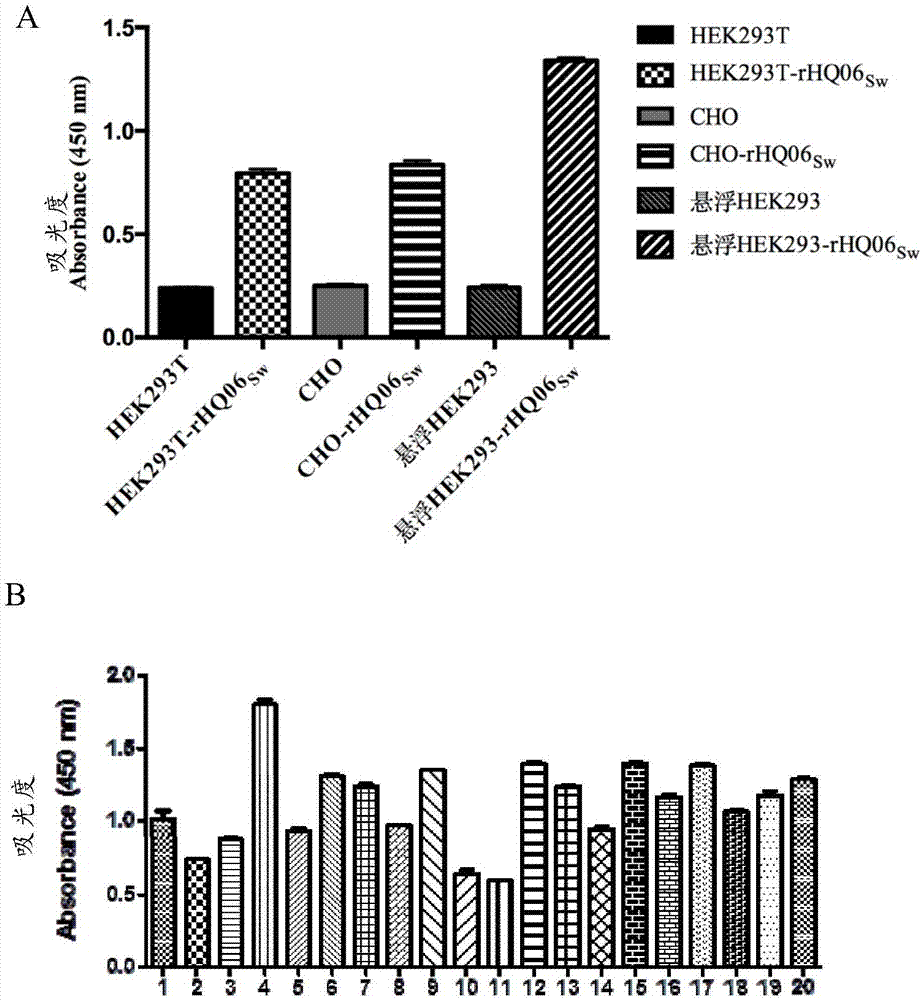
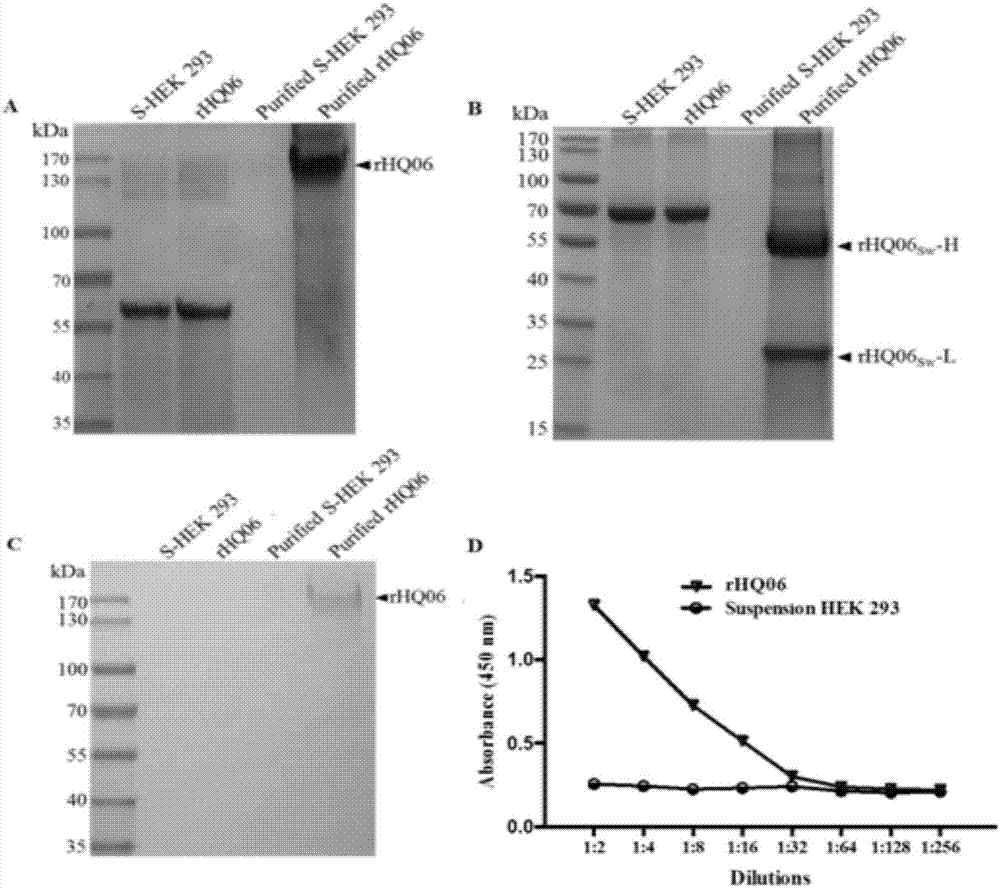
[0045]Human embryonic kidney cells (HEK293T) and pig kidney cells (PK-15) were purchased from the American Type Culture Collection; Chinese hamster ovary cells (CHO) were purchased from the Cell Bank of the Chinese Academy of Sciences; suspension HEK293 cells were purchased from Harbin National Engineering Research Institute for Animal Biological Products Provided by Center Ltd. CSFV Shimen strain is preserved by our laboratory. Anti-CSFV E2 protein monoclonal antibody WH303 was donated by Professor Trevor W.Drew from the National Veterinary Laboratory of the United Kingdom. BVDV porcine-derived positive serum was donated by Professor Paul Becher of the OIE / EU Reference Laboratory for Swine Fever at Hannover Veterinary University.
[0046] HEK293T and PK-15 cells were cultured with DMEM containing ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com