Monoclone antibody and application thereof
A monoclonal antibody and antibody technology, applied in the direction of antibodies, antiviral agents, antiviral immunoglobulins, etc., can solve the problems of cumbersome operation and low monosensitivity, so as to improve accuracy and make up for prevention and/or treatment. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0023] As an embodiment of the present invention, the antibody is monoclonal antibody 15A9, the amino acid sequence of the heavy chain variable region of the monoclonal antibody 15A9 is SEQ ID No. 2, and the amino acid sequence of the light chain variable region is SEQ ID No. 2. ID No.4.
[0024] As an embodiment of the present invention, the antibody is monoclonal antibody 15A9, and the amino acid sequence of the heavy chain variable region of the monoclonal antibody 15A9 is the nucleotide sequence shown in SEQ ID No.1 or its degenerate sequence The coding, and the amino acid sequence of the light chain variable region are coded by the nucleotide sequence shown in SEQ ID No.3 or its degenerate sequence.
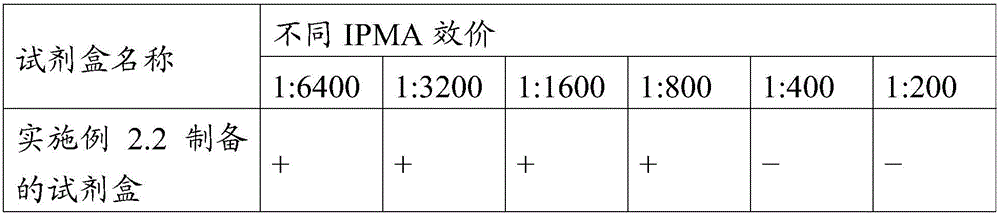
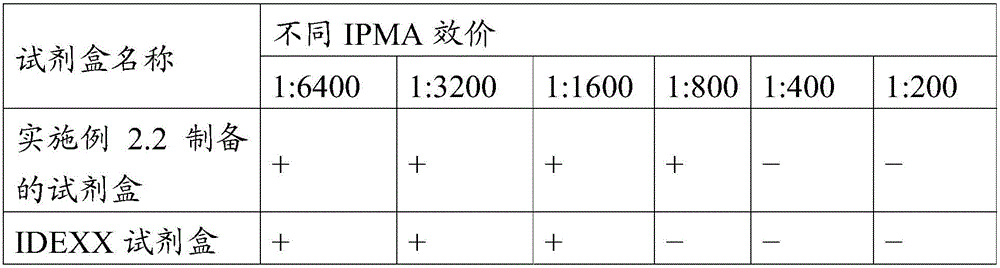
[0025] Described monoclonal antibody 15A9 is anti-swine fever virus monoclonal antibody 15A9, and its neutralizing activity titer is 1:6400, has good neutralizing activity; IPMA (immunoperoxidase monolayer cell test) titer is 1:6400 12800, has good reactivity with classical...
Embodiment 1
[0060] Example 1 Preparation, purification and identification of monoclonal antibody against classical swine fever virus
[0061] 1.1 Preparation of CSFV antigen and determination of its content
[0062] Refer to He Yan (He Yan. The expression of classical swine fever virus E2 protein in insect cells and the establishment of indirect ELISA antibody detection method. 2008, Nanjing Agricultural University master's degree thesis) literature operation method to prepare the classical swine fever virus antigen that is E2 protein; BCA Protein Concentration Determination Kit (purchased from Shanghai Biyuntian Biotechnology Co., Ltd.) was used to determine the concentration of E2 protein, and the concentration was 1.1 mg / ml.
[0063] 1.2 Preparation and purification of monoclonal antibody against CSFV
[0064] The E2 protein of classical swine fever virus was immunized into mice according to the amount of 100 μg / 200 μl, according to the operation of Harlow E et al. (Harlow E, Lane D. ...
Embodiment 2
[0090] Example 2 Preparation, detection method and application of swine fever virus ELISA antibody detection kit (blocking method)
[0091] 2.1 Preparation and content determination of enzyme-labeled monoclonal antibody 15A9
[0092] Under dark conditions, use the modified sodium periodate method to label the monoclonal antibody with HRP: Weigh 20mg of horseradish peroxidase (HRP) and dissolve it in 1ml of ultrapure water, add 1ml of freshly prepared NaIO 4 Solution (specifically 20mg NaIO 4 Dissolve in 1ml ultrapure water), mix well, and protect from light at 4°C for 30 minutes; add 20 μl of ethylene glycol solution to the above solution, and react at 4°C for 30 minutes; Add 100 μl of the above mixture, mix the two, add to the dialysis bag and mix, and dialyze with carbonic acid buffer for 6 hours; transfer the dialyzed mixture to a 1.5ml EP tube, add 10 μl of freshly prepared NaBH 4 Solution (specifically 10mg NaBH 4 Dissolve in 1ml ultrapure water), act for 2 hours at ro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com